Question
Question: What law of thermodynamics explains why only a fraction of the energy ingested by organism A is used...
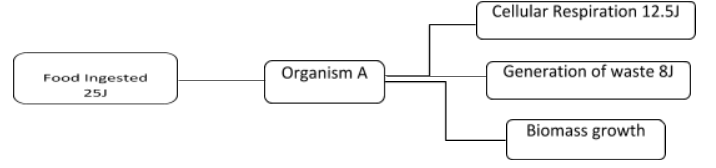
What law of thermodynamics explains why only a fraction of the energy ingested by organism A is used in making new cells or growing?
 A. The first law of thermodynamics, which states that matter is never created or destroyed.
A. The first law of thermodynamics, which states that matter is never created or destroyed.
B. The first law of thermodynamics which deals with the conservation of energy.
C. The second law of thermodynamics, which explains that all systems seek entropy.
D. The second law of thermodynamics which deals with the conservation of momentum in a physical system.
Solution
The first law of thermodynamics, one form of energy is converted into another form of energy. The second law states that the total entropy of the system can never decrease.
Step by step answer: The first law of thermodynamics defines the law of conservation of energy. According to the law of conservation of energy, the total energy of an isolated system remains constant. It also means energy can neither be created nor can be destroyed but can only be transformed or transferred from one form of energy to another form of energy. For example, when a stick of dynamite explodes, chemical energy is converted into kinetic energy.
The food ingested by an organism is used for various cellular level activities like cellular respiration, ATP generation, waste generation, and also lost as heat. So, here the energy is distributed for all the purposes and not utilized only for cell growth and development.
Therefore, from all the options provided above, option B: the first law of thermodynamics which deals with the conservation of energy is the appropriate and correct answer.
Note: Conservation of energy for some systems which do not have time translation symmetry. A mathematical transformation in physics that moves the times of events through a common interval is called time translation symmetry. The Law of conservation of energy is not only applicable for living organisms but it is used in chemistry, physics, mathematics, quantum theory, and mechanics. However, conservation of mass is different from the conservation of energy.
