Question
Question: What is \( X \) in the following reaction? 
(A) CH3OH,H2SO4
(B) CH3OH,CH3O−Na+
(C) H2O/H2SO4 followed by CH3OH
(D) CH3MgBr/ether followed by H3O+
Solution
Epoxides are cyclic ethers, in which the oxygen atoms and alkyl groups were arranged in a cyclic form. When the epoxides are treated with sulphuric acid in presence of methanol, the bond between the oxygen and adjacent carbon will break leading to the formation of corresponding alcohol.
Complete Step By Step Answer:
Chemical compounds are classified into functional groups based on the groups or molecules present in the compound. When an oxygen atom is present in between the two alkyl groups. Then that compound can be known as ether. When ether is arranged in a cyclic form, then those compounds were known as epoxides.
Given that a cyclic ether is treated with a chemical reagent X it leads to the cleavage of oxygen and carbon bonds and forms corresponding alcohol.
When epoxides are treated with methanol in presence of a strong acid like sulphuric acid. The hydrogen atom can be lost from the methanol that generates a nucleophile. Due to the electronegativity of oxygen, it attracts the electron from the adjacent tertiary carbon. Now, these nucleophile attacks on the epoxide lead to the formation of the below product.
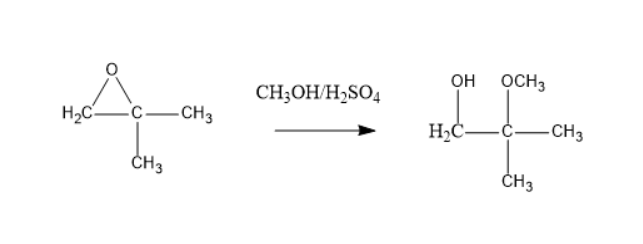
Thus, X is CH3OH,H2SO4
Option A is the correct one.
Note:
The oxygen atom in epoxide attracts the electrons from the adjacent tertiary carbon atom only, but not the adjacent primary carbon atom. As the tertiary carbocation is more stable than the primary carbocation. Finally, based on the cleavage of carbon-oxygen the alcohol was formed.
