Question
Question: What is W? 
A.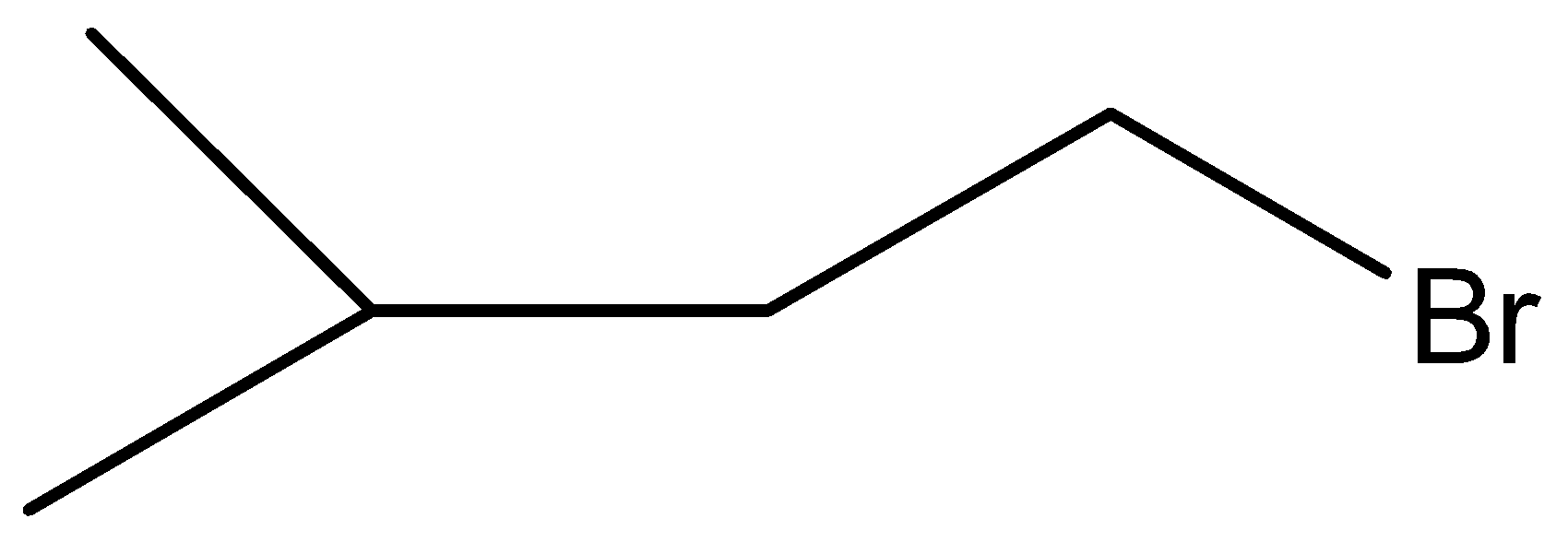
B.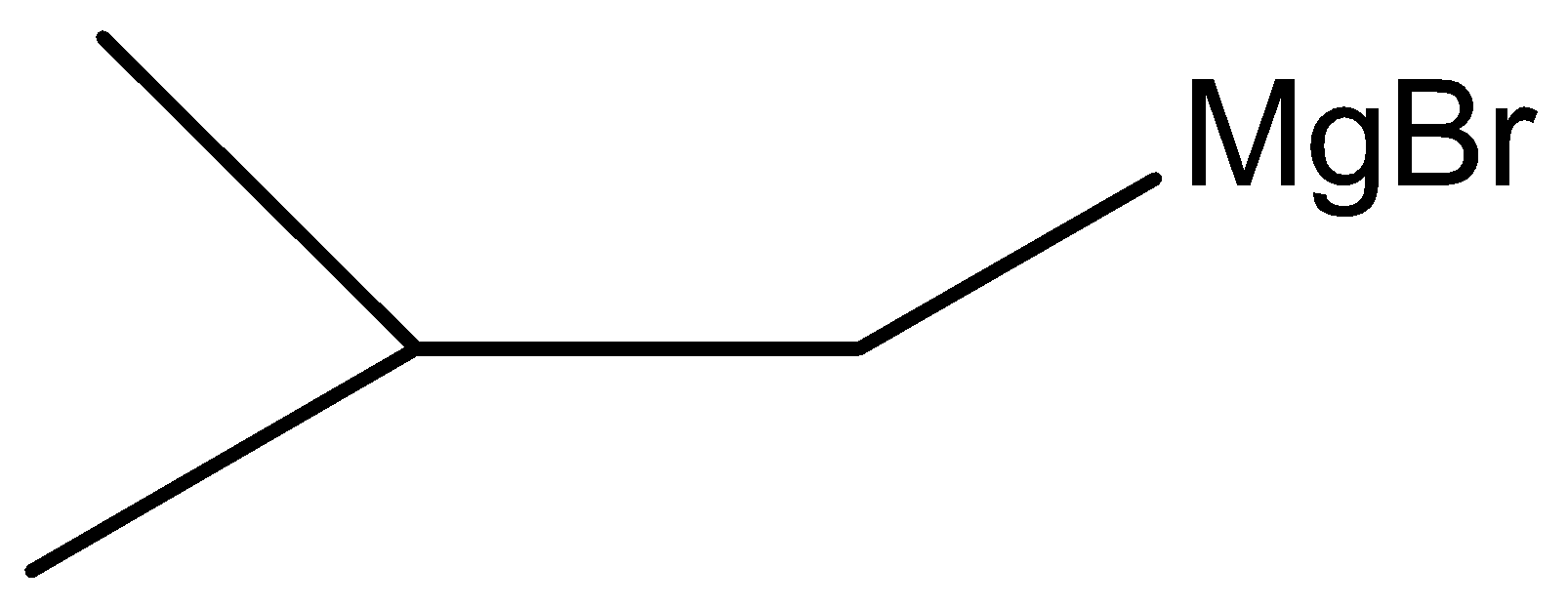
C.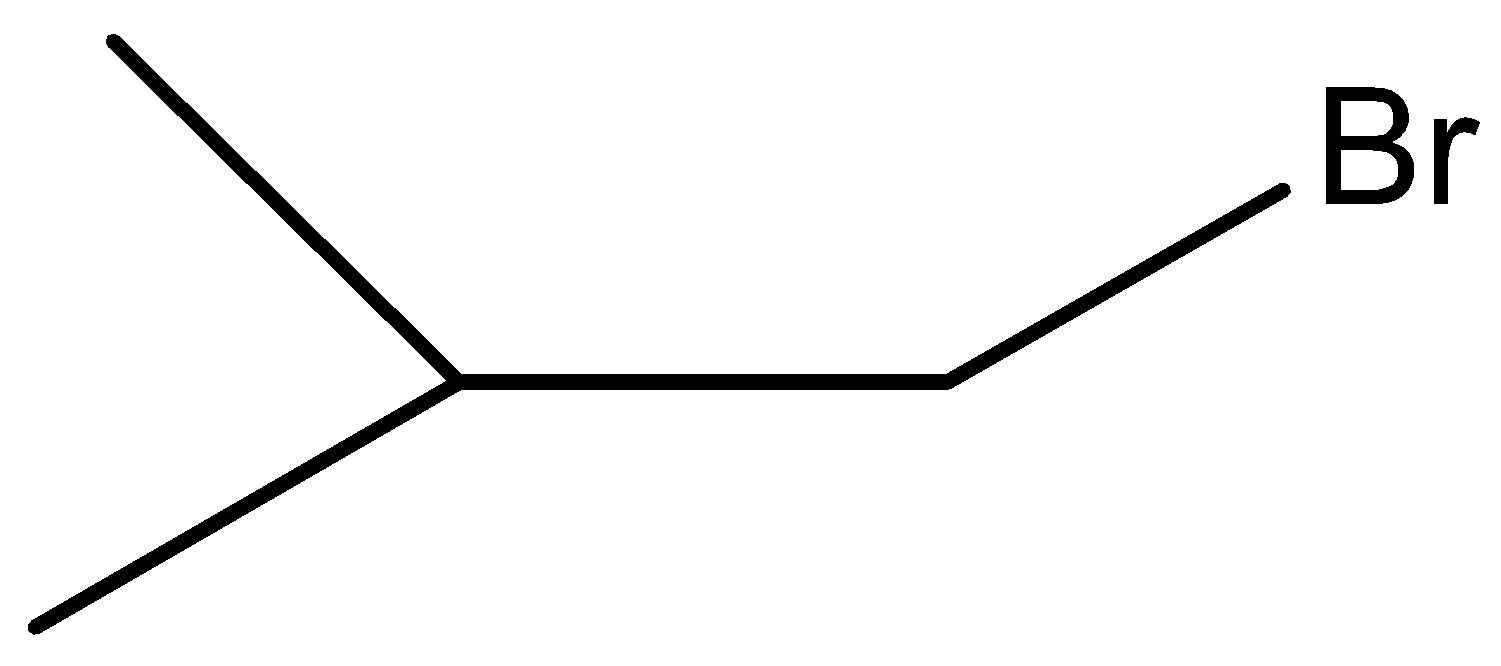
D.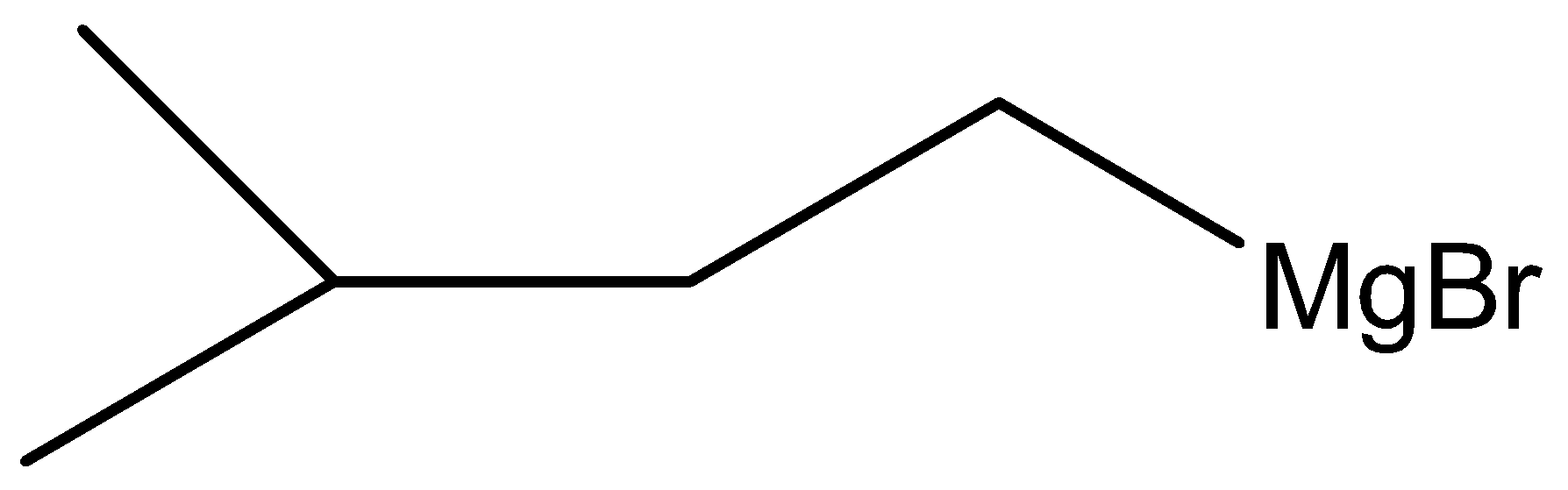
Solution
A Grignard reagent has a formula RMgX where X is a halogen, and R is an alkyl or aryl (based on the benzene ring) group. A typical Grignard reagent might be .
Complete answer:
They can be used for the synthesis of a wide range of organic compounds and are also very useful to the organic chemist.
Grignard reagents are formed by adding the halogenoalkane to a small amount of magnesium in a flask containing ethoxyethane which is commonly called diethyl ether or just ether. The flask is used with a reflux condenser, and the mixture is heated over a water bath for 20 - 30 minutes. The organ magnesium compounds made by the reaction of an alkyl or aryl halide with magnesium are called Grignard reagents. Grignard reagents are used to synthesize a variety of organic compounds and are extremely important to the organic chemist. The highly basic nature of a Grignard reagent usually results in an elimination reaction or no reaction at all. The transition state to add the alkyl halide is less stable than the Magnesium/Bromide(Halide) complex. This is because of a ligation formation between the solvent and the Magnesium atom.
So, the reaction is
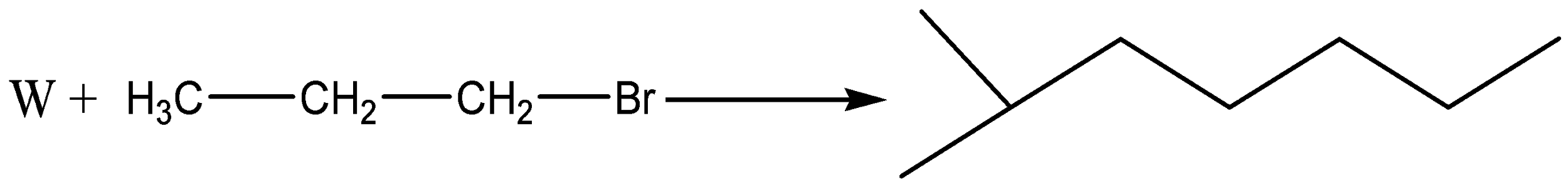
Here, W is 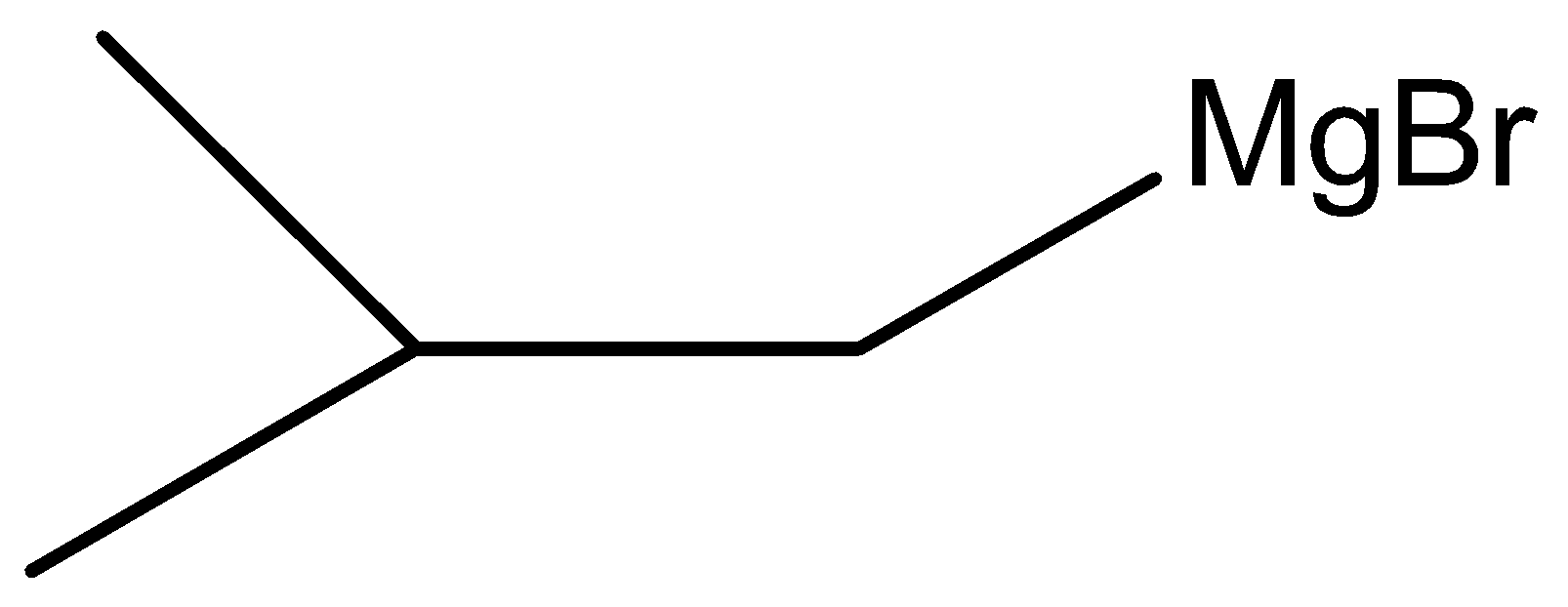
Therefore, the correct answer is option (B).
Note: 1-Bromopropane is a colourless liquid which was originally used in the production of pesticides, flavours, fragrances, pharmaceuticals, and other chemicals. The commercial 1-bromopropane includes not only 1-bromopropane, but also additives that improve its performance in the desired application and stabilizers to inhibit decomposition. It has a role as a neurotoxin and a solvent. It is a bromoalkane and a bromo hydrocarbon.
