Question
Question: What is \(\vartriangle \text{U}\) for the presence described by the figure? Heat supplied during t...
What is △U for the presence described by the figure?
Heat supplied during the process, q= 100 KJ.
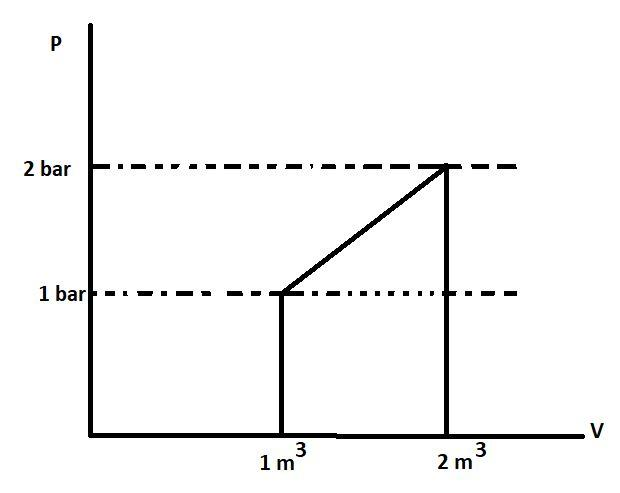
A. +50 KJ
B. -50 KJ
C. -150 KJ
D. +250 KJ
Solution
Find the work done using the P-V graph; as the area under the P-V curve represents the work done by the gas. Then use the first law of thermodynamics to calculate △U. The first law says that △U=W+Q. Do convert the units into joules.
Complete answer:
Let us first find the work done by the gas using the given graph:
We know that the area under the P-V curve gives the work. This is because the formula of work done is P△V. The area or work in the P-V graph is represented by :
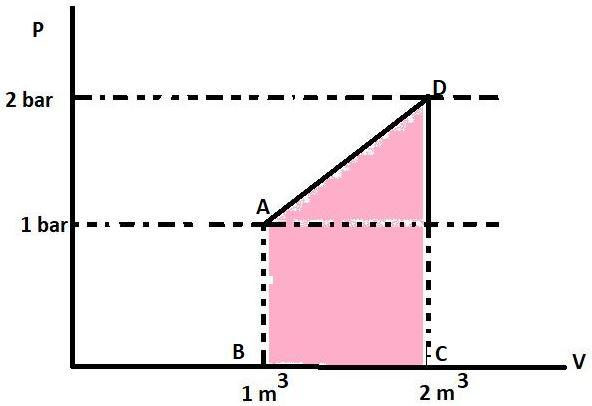
This coloured portion is a trapezium with AB and CD as its parallel sides and height between the two is △V. The area of trapezium is sum of parallel sides multiplied to the height and divided by 2 or 21×(Sum of parallel sides)×(Height). The parallel sides have the value 1 bar and 2 bar respectively. The height is △V or (2-1) m3 . So, the area will be 21×(1+2)×(1) or 1.5 bar-m3. The work in joules will be 150 KJ as 1 bar-m3=100 KJ. Work is positive because △V is positive. So, the value of W is +150 KJ.
- First law of Thermodynamics says that energy can only be converted from one form to another. It can neither be destroyed nor be created. Mathematically, its expression is △U=W+Q.
Q= + 100 KJ, positive because it is added to the system from outside. Using the formula, △U will be 100+150 or 250 KJ.
The change in internal energy or △U for the process is +250 KJ.
The correct option is ‘d’.
Note:
The unit of work done should be known which is joule. The conversion units have to be used correctly. It should be known that if △V is positive, then work will be positive and if △V is negative then, work will also be negative.
