Question
Question: What is true about \(N{O_2}\)and\(Cl{O_2}\)? The question has multiple correct answers. A.Both m...
What is true about NO2andClO2?
The question has multiple correct answers.
A.Both molecules have fractional bond order.
B.Both oxides are neutral in nature.
C.Both have odd electron bond in their structure
D.Both are paramagnetic in nature.
Solution
Para magnetism is due to the presence of some unpaired electron in the molecule. The molecules of paramagnetic compounds generally contain odd numbers of electrons or odd-electron molecules. When the total number of electrons present in the molecule is odd, at least one of the electrons has to remain unpaired, meaning one electron remains single.
Complete Step by step solution:
Nitrogen dioxide has twenty three electrons and carbon dioxide has thirty three electrons. These are Paramagnetic species because both have acid electrons.
Nitrogen dioxide is paramagnetic which is mostly ready because as it does not contain alpha orbits so the odd electron of nitrogen in Nitrogen dioxide gets removed or delocalized. So nitrogen dioxide gets divided. But chlorine dioxide does not give blue due to the reason that the odd electron is delocalized as it is involved in pπ=απ bonding.
The nitrogen dioxide has hybridization p2 because it has planar structure and in chlorine dioxide we have two bonds, one some pair bond and one singlet bond. So it has hybridization of sp2 .
Nitrogen dioxide has trigonal planar structure because one s and two p orbital are involved in bonding which leads to a bendy structure of nitrogen dioxide. Whereas chlorine dioxide doesn’t have any bendy shape as shown below:
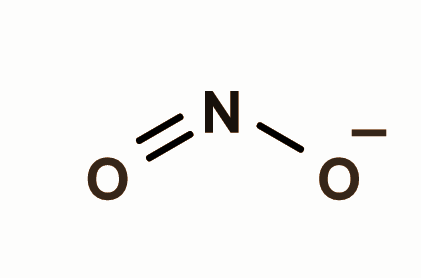
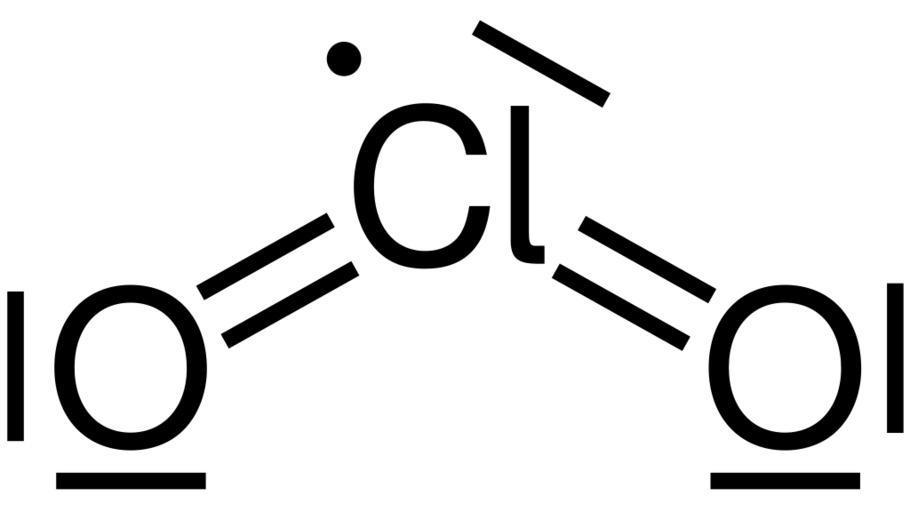
So the correct answer is B and D.
Additional Information
Chlorine dioxide is a yellowish green gas which acts as an oxidizing agent. If we add it in water it does not hydrolyze and act as a dissolved gas in solution in water. It is commonly used as a bleach. It is also hazardous including health concerns and fire ignition.
Nitrogen oxide or dioxide is an industrial product of nitric acid. It is mostly produced in the field of fertilizers. It is a paramagnetic bent molecule and point group symmetry shape. It is used as a temperature sterilization agent and also as fuel in rockets. Mostly its sources are from combustion of engines which burns fossil fuels for example from motor vehicles.
Note: Nitrogen dioxide is likewise a forerunner in the arrangement of nitrate aerosols and nitrosamines, the wellbeing impacts of which are under examination. On account of the amount created and their potential for far and wide unfavorable impacts on general wellbeing and government assistance, nitrogen oxides are among the barometrical toxins for which norms and routinely controls have been set up both by the U.S.C.
