Question
Question: What is the wavelength of a photon emitted during a transition from \(n = 5\) state to \(n = 2\) sta...
What is the wavelength of a photon emitted during a transition from n=5 state to n=2 state in the hydrogen atom?
A. 434nm
B. 450nm
C. 350nm
D. 525nm
Solution
Hint: The photon emitted during the transition from any state to n=2 state lies on Balmer series of transition. When there is a transition between the two states in a hydrogen atom, the relation between the number of the states and the wavelength is given by the Rydberg equation for hydrogen atom. By using that relation, the wavelength of the photon can be calculated.
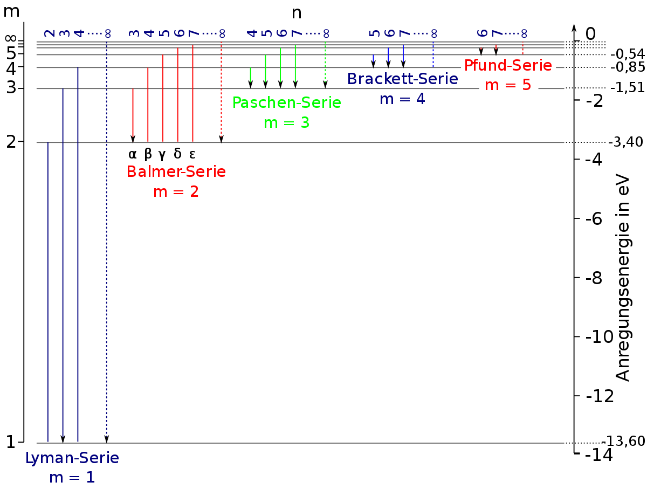
Useful formula:
The Rydberg equation for hydrogen atom is given by,
λ1=RH[n121−n221]
Where, λ is the wavelength of the emitted photon, RH is the Rydberg constant of hydrogen, n2 is the initial state of transition and n1 is the final state of transition.
Complete step by step solution:
Given, The final state of transition, n1=2
The initial state of transition, n2=5
The Rydberg constant of hydrogen, RH=10973731.6m−1
The Rydberg equation for hydrogen atom is given by,
λ1=RH[n121−n221].....................................(1)
Substitute the given values of n1, n2 and RH in the equation (1),
λ1=10973731.6m−1[221−521] λ1=10973731.6[41−251] λ1=10973731.6[0.25−0.04] λ1=10973731.6[0.21] λ1=2304483.636m−1
Taking reciprocal on both sides,
λ=2304483.636m−11 λ=4.3393×10−7m
Convert unit from m to nm,
1m=109nm
Hence,
λ=4.3393×10−7m×1m109nm λ=4.3393×102nm λ=433.93nm λ≃434nm
Hence, the wavelength of photon emitted during transition, λ=434nm
Thus, the option (A) is correct.
NOTE:
Alternative method:
Hint: The energy level of photons in each level are calculated by using the energy formula for each state. Then the difference in the energy is obtained by taking the difference between two energies. And by using the Planck-Einstein relation the frequency of the emitted photon can be calculated and with the help of the relation between speed of light, wavelength and frequency of light, the wavelength can be obtained.
Useful formula:
The energy of hydrogen in each state is given by,
En=−n213.6eVJoules
Where, −13.6eV is the approximate ground state energy of the hydrogen atom and n is the state.
The Planck-Einstein relation is given by,
E=h×υ
Where, E is the energy difference, h is the Planck’s constant and υ is the frequency of the photon.
The relation between speed of light, wavelength and frequency of light is given by,
c=λ×υ
Where, c is the speed of photon or light, λ is the wavelength of photon and υ is the frequency of photon.
Complete step by step solution:
Given, The final state of transition, n1=2
The initial state of transition, n2=5
Planck’s constant, h=6.626×10−34J.s
Speed of photon, c=3×108sm
Step by step solution:
The energy of hydrogen in n=5 state,
E5=−5213.6eV E5=−2513.6 E5=−0.544eV
Convert eV to J,
1eV=1.602×10−19J
Hence,
E5=−0.544×1.602×10−19J E5=−0.871×10−19J E5=−8.71×10−20J
The energy of hydrogen in n=2 state,
E2=−2213.6eV E2=−413.6 E2=−3.4eV
Convert eV to J,
1eV=1.602×10−19J
Hence,
E2=−3.4×1.602×10−19J E2=−5.44×10−19J
Difference in energy between n=5 state and n=2 state,
ΔE=E2−E5...............................(2)
Substitute the values of E2 and E5 in equation (2),
ΔE=(−5.44×10−19J)−(−8.71×10−20J) ΔE=4.57×10−19J
The Planck-Einstein relation is given by,
ΔE=h×υ...........................(3)
Substitute the values of ΔE, h and υ in equation (3),
4.57×10−19J=6.626×10−34Js×υ υ=6.626×10−34Js4.57×10−19J υ=6.897×1014s−1
The relation between speed of light, wavelength and frequency of light is given by,
c=λ×υ.................................(4)
Substitute the values of cand υ in equation (4),
3×108ms−1=λ×6.897×1014s−1 λ=6.897×1014s−13.8×108ms1 λ=4.354×10−7m
Convert unit from m to nm,
1m=109nm
Hence,
λ=4.35×10−7m×1m109nm λ=4.35×102nm λ=435nm λ≃434nm
Hence, the option (A) is correct.
These two methods are used to find the wavelength of the photon emitted from one state to another state. In the first method the Rydberg equation is directly used to find the wavelength and in the second method, the energy equation, Planck-Einstein equation and relation between speed, wavelength and frequency of photon are used. Bothe these methods give the approximate answer of wavelength.
