Question
Question: What is the VSEPR model for \( CHC{l_3} \) ?...
What is the VSEPR model for CHCl3 ?
Solution
Hint : VSEPR is used to predict the shapes of molecules by following a systematic way with the help of numbers of electron pairs to determine the shape of the molecule. And to know how many electrons are there in the outer shell just check it belongs to which group and the group number will be equal to valence electrons.
Complete Step By Step Answer:
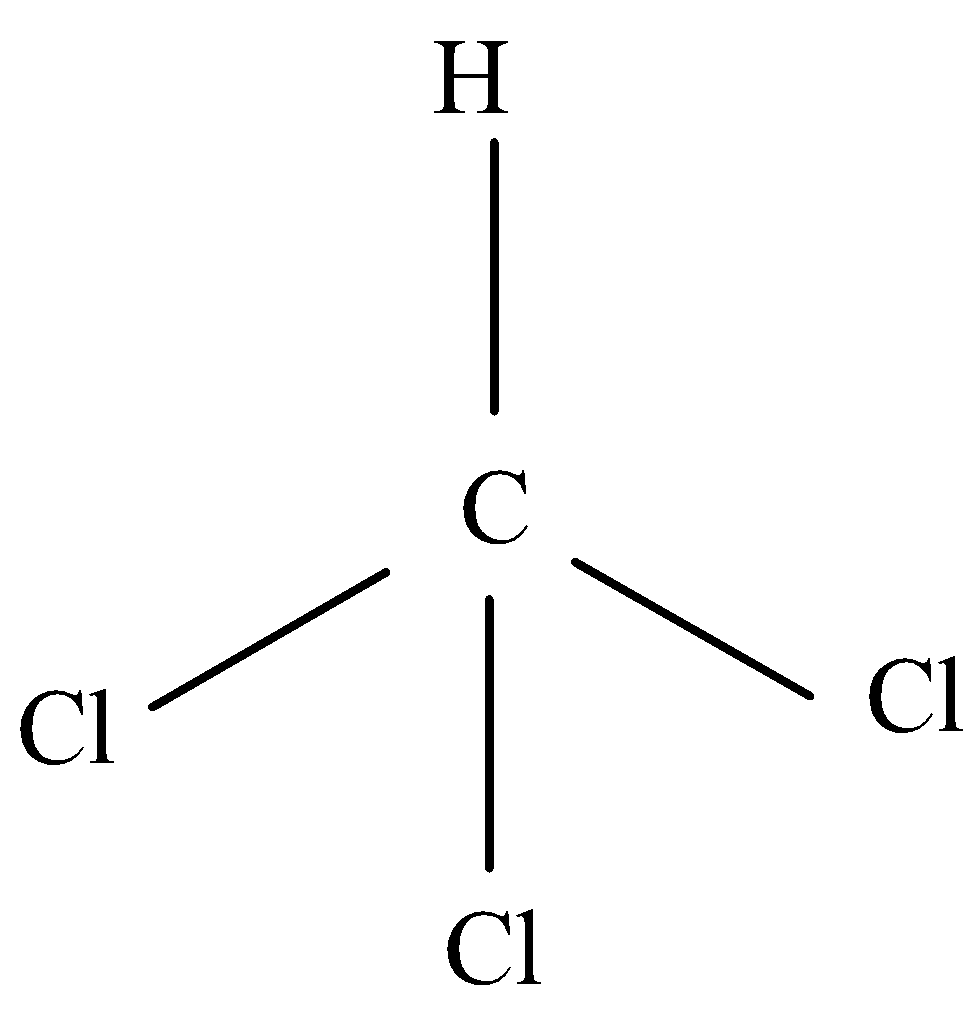
Let's go step by step, first identify the central atom, now the central atom will be C. Then we will count its valence electrons that are four. Now we will add each electron to each bonding atom. Sometimes we will have to add or subtract electrons for charge. Divide the total of these by two to get the total number of electron pairs and then we will use this number to predict the shape.
There would be three chlorine atoms and a single bond to hydrogen where chlorine will have a single covalent bond. By following all steps, we come to the conclusion that CHCl3 will have AX4 designation. That is, it will make a tetrahedral shape.
Note :
Full form of VSEPR is valence shell electron pair repulsion. It basically states that bonding and nonbonding electron pairs of the central atom in a molecule will push each other away in 3D space and molecules will have their own shapes.
