Question
Question: What is the VSEPR model for \(C{{H}_{2}}O\)?...
What is the VSEPR model for CH2O?
Solution
The Valence Shell Electron Pair Repulsion Theory states that the atoms around the central atom will arrange in such a way that there is minimum repulsion between valence electron pairs of the atom.
Complete answer: We know that CH2O is the molecular formula for formaldehyde or methanal.
To find out the VSEPR model of CH2O, we first need to draw its Lewis dot structure.
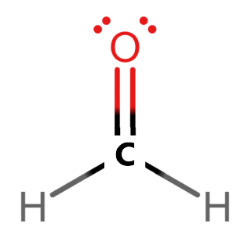
Here from the Lewis dot structure, we can see that the carbon atom is surrounded by three electron cloud regions. It forms two single bonds with hydrogen and one double bond with oxygen which has 2 lone pairs.
Or we can say that the carbon atom has four bond pairs and no lone pairs and hence the formaldehyde molecule CH2O is a AX3 species.
According to the VSEPR theory, atoms are arranged around the central atom in such a way that the molecule has maximum stability and minimum energy.
So, these electron cloud regions will be the furthest apart from each other when they are separated by an angle of 120∘ on a plane to give maximum stability and minimum energy and hence will form a compound having a trigonal planar shape.
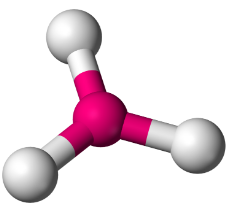
Note: It should be noted that there is maximum repulsion between two lone pairs and minimum repulsion between two bond pairs. Hence, when there are lone pairs on the central atom of the molecule, the shape of the molecule is different from predicted and has a distorted shape.
