Question
Question: What is the value of \(\Delta {H_{AB}}\) \(\left( {in\;kJ} \right)\) for a fluid in the given proces...
What is the value of ΔHAB (inkJ) for a fluid in the given process represented in the figure? Heat supplied during the process =2740kJ.
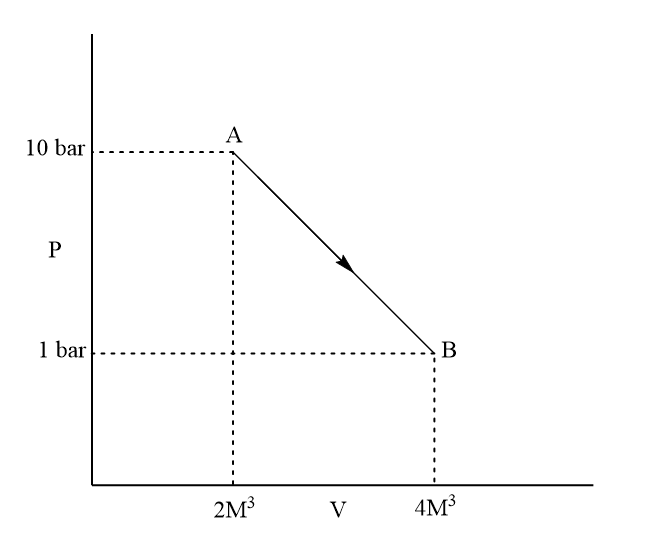
A.40kJ
B.400kJ
C.1640kJ
D.None of these
Solution
When a process takes place under constant pressure, the change in Enthalpy is equivalent to the heat absorbed or released. Enthalpy is also termed as “heat content”. The word enthalpy is a Greek word meaning ‘warming”.
Complete answer:
Enthalpy is summation of internal energy(U) and the product of pressure(P) and volume(V). The formula of enthalpy is:
H=U+pV
Where, H=Enthalpyofthesystem
U=Internalenergyofthesystem
p=Pressure of the system
V = Volume of the system
We cannot measure enthalpy directly but the change in enthalpy (ΔH)can be measured which is the loss or addition of heat in the system. It always depends upon T, p and U.
The formula of change in enthalpy is:
ΔH=ΔU+ΔPV
Entropy and enthalpy are different. They can be used interchangeably. But there is a great difference in both of them.
| S.No. | Entropy | Enthalpy |
|---|---|---|
| 1 | Entropy is the property which helps in the measurement of the movement of molecules. | Enthalpy is the energy which is composed of both internal energy and energy flow. |
| 2 | It is regarded only under limitations or controlled conditions. | It is measured or applicable under normal or regular conditions. |
| 3 | Joule per Kelvin is the unit of measurement. | Joule per mole is the unit of measurement. |
| 4 | The maximum amount of Entropy is favoured by the thermodynamic system. | The minimum amount of Enthalpy is favoured by the thermodynamic system. |
Here,
PΔV=1bar×2m3
PΔV=2barm3
PΔV=200000J
=200kJ
ΔH=−2740kJ+200kJ
ΔH=−2540kJ
Thus, the right answer is the (d) option, none of these.
Note:
If the heat given out by the system into the surrounding, it is called an exothermic reaction and the heat given to the system from the surrounding is known as endothermic reaction. Exothermic reactions don’t contain much energy within them.
