Question
Question: What is the type of hybridisation of each carbon in the following compounds? \((a)C{H_3}Cl\) \((...
What is the type of hybridisation of each carbon in the following compounds?
(a)CH3Cl
(b)(CH3)2CO
(c)CH3CN
(d)HCONH2
(e)CH3CH=CHCN
Solution
Hybridisation can be defined as the mixing of valence orbitals which lead to the formation of sigma bonds. The types of orbitals that are included in mixing and the number of sigma bonds formed helps to determine the hybridisation state of carbon in an organic compound. In the solution we will see how the number of sigma bonds formed by the carbon atom determines its hybridisation state.
Complete answer:
We know that hybridisation state can be defined as the mixing of valence orbitals which lead to the formation of sigma bonds. The types of orbitals that are included in mixing and the number of sigma bonds formed helps to determine the hybridisation state of carbon (central atom) in any given organic compound.
Now, we will see how sigma bonds relate to the hybridisation of carbon atoms.
| No. of sigma bonds that carbon forms | Hybridisation state |
|---|---|
| 4 | sp3 |
| 3 | sp2 |
| 2 | sp |
Therefore, on the basis of above table we will determine the hybridisation state of carbon atom in each of the given compounds:
(a)CH3Cl
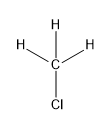
In this compound, carbon forms four sigma bonds. Hence, the hybridisation state of carbon is sp3 .
(b)(CH3)2CO
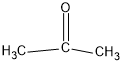
In this compound, carbon forms three sigma bonds. Hence, the hybridisation state of carbon is sp2 .
(c)CH3CN
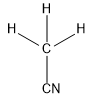
In this compound, carbon forms four sigma bonds. Hence, the hybridisation state of carbon is sp3 .
(d)HCONH2
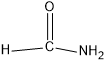
In this compound, carbon forms three sigma bonds. Hence, the hybridisation state of carbon is sp2 .
(e)CH3CH=CHCN
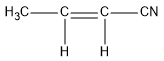
In this compound, carbon at the end forms four sigma bonds. Hence, the hybridisation state of that carbon is sp3 . The two middle carbon atoms have three sigma bonds and the hybridisation state is sp2 . The carbon attached to nitrogen atom forms two sigma bonds and hybridisation state of this carbon is sp.
Note:
This is how we find the hybridisation state of carbon in organic compounds. For inorganic compounds, to find the hybridisation state of the central atom, we need to know the valence shell configuration of the central atom, the monovalent ions present in the compound and the cationic or ionic charge present on the compound.
