Question
Question: What is the total number of \(S - S\) bonds in polythionic acid? (A) \(n\) (B) \(n - 1\) (C) \...
What is the total number of S−S bonds in polythionic acid?
(A) n
(B) n−1
(C) n−2
(D) n−3
Solution
As we know that polythionic acid contains a straight chain compound of sulphur atoms having the formula H2SnO6 where the number of sulphur atoms can differ in every compound formed and they are basically the conjugate acids of polythionates which are commonly available rather than polythionic acids that are rare.
Complete answer: As we know that polythionic acids are basically the conjugate acids of polythionates which are common and important rather than the polythionic acids which are rarely encountered. They contain a straight chain of sulphur atoms and are attached with two hydrogen and six oxygen atoms.
We should know that Polythionic acids have a chemical formula which is H2SnO6 where the number of sulphur atoms may vary in compounds formed. The basic condition is that the number of sulphur atoms should be more than two.
For instance: H2S3O6 is called the trithionic acid and the number of sulphur in this compound is three, so the S−S bonds in this compound will also be 2. Similarly we have tetrathionic acid with a chemical formula H2S4O6, so it will have 3.
So we can say that the skeletal formula of any polythionic acid can be given as:
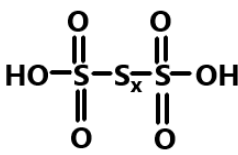
Hence, in conclusion we can say that all the polythionic acids are named through the number of atoms in the straight chain of sulphur atoms. And the basic formula to calculate the number of sulphur bonds is n−1.
Therefore the correct answer is (B).
Note: Remember that a clear mechanism is not clear about the manufacturing or synthesis of polythionic acids. But, the reaction between hydrogen sulphide and sulphur dioxide in aqueous solution can yield a mixture of oxyacids of sulphur having different structure. Most stable polythionic acids are those having small numbers of sulphur atoms in the chain.
