Question
Question: What is the total number of lone pair of electrons in \[{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{3}...
What is the total number of lone pair of electrons in N2O3?
Solution
Hint : Lone pair of electrons in N2O3 can be determined by drawing its Lewis structure. Lewis structure can be made by counting total valence electrons present in N2O3.
Complete step by step solution :
In order to calculate lone pair of electrons in N2O3, first we need to draw its Lewis structure. Lewis structure can be made by using the total number of valence electrons needed by all atoms to achieve noble gas configuration.
In N2O3, there are two nitrogen atoms and three oxygen atom. Thus the total number of valence electrons (V.E) can be calculated as follows:
Totalvalenceelectrons=2(V.EofN)+3(V.EofO)
Since we know that the atomic number of nitrogen is 7 and its electronic configuration is 2, 5. This shows that there are 5 electrons in the outermost shell (valence shell). Thus the valence number of nitrogen is 5. The atomic number of oxygen is 8 and its electronic configuration is 2, 6. This shows that there are 6 electrons in the outermost shell. Thus the valence number of oxygen is 6.
Hence the total number of valence electrons in N2O3 can be calculated as:
{\text{Total}}\;{\text{valence}}\;{\text{electrons}} = \left( {2 \times 5} \right) + \left( {3 \times 6} \right) \cr = 10 + 18 \cr = 28 \cr} $$ This means that there are a total 28 valence electrons needed by all atoms to acquire noble gas configuration. Nitrogen is the least electronegative element. Thus two nitrogen atoms will be placed in the centre and then we will put three oxygen around them. We will put electrons between atoms to produce chemical bonds. When each atom acquires complete octet, Lewis structure is ready as follows: 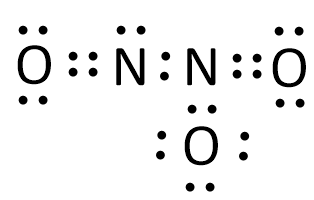 In the above structure, each shared pair of electrons is treated as a single bond which is represented by a single line. Double shared pairs of electrons are treated as double bonds which are represented by two double parallel lines. Thus the above structure can also be represented as: 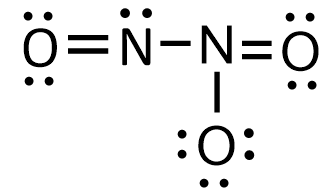 Lone pairs are those electron pairs which do not participate in bond formation. From the above structure, it can be observed that there are a total eight electron pairs which are not participating in the bond formation. Thus there are total 8 lone pairs in $${{\text{N}}_{\text{2}}}{{\text{O}}_{\text{3}}}$$. **Note** : Each shared pair of electrons is known as a bond pair and each unshared pair of electrons is known as a lone pair. Bond pairs of electrons participate in the bond formation while lone pairs of electrons do not participate in the bond formation.