Question
Question: What is the sulphuric acid obtained from ferrous sulphate (green vitriol) fumes strongly in moist ai...
What is the sulphuric acid obtained from ferrous sulphate (green vitriol) fumes strongly in moist air, on account of the long-continued practice of the process at Nordhausen called?
A. Fuming sulphuric acid
B. Nordhausen sulphuric acid
C. Vitroling sulphuric acid
D. Both A and B
Solution
It is found that historically the biggest production of Fuming sulphuric acid came from distillation of iron sulphates at Nordhausen, from which the historical name Nordhausen sulphuric acid is derived. The structure of Fuming sulphuric acid is:
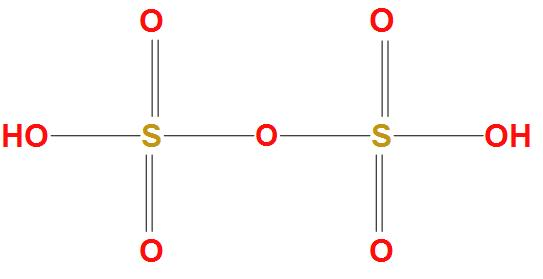
Complete Solution :
- Let’s discuss about the preparation of Fuming sulphuric acid:
It is found that Fuming sulphuric acid is prepared by the dry distillation of sulphate of iron. (FeSO4.7H2O) is firstly gently heated in the air till all the water is expelled completely and the part of ferrous sulphate is oxidized to ferric state.
- In the next step the mixture of sulphates is then kept in small iron retorts, which are heated in a fumace. The decomposition takes place in accordance with the following equation:
