Question
Question: What is the structure of \({K_3}Cr{O_8}\) and what is the oxidation number of Cr here?...
What is the structure of K3CrO8 and what is the oxidation number of Cr here?
Solution
The chemical name of K3CrO8 is Potassium tetraperoxochromate. As the chemical name suggests that there are four peroxy linkages present in the molecule. Peroxy here states that two oxygen bonds are joined by a single bond. In the solution we will see the structure of this compound and the oxidation state of chromium by the substitution method.
Complete answer:
We are given a chemical compound whose chemical formula is K3CrO8 . The chemical name for this compound is Potassium tetraperoxochromate.
The structure of this compound is given as:
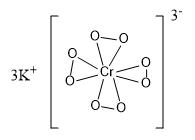
In the above structure we can see that there are four peroxy linkages present in the compound. The structure of the given compound is tetragonal.
Oxidation state of any atom is referred to as the total number of electrons it loses or gains in order to form bonds with other atoms.
Let the oxidation state of Chromium, Cr be x.
Oxidation state of Oxygen is −2 and the charge on complex is −3.
We will now form an equation to calculate oxidation state of chromium:
4×(−2)+x=−3
On solving this, we get
x=+5
Therefore, oxidation state of Chromium, Cr in K3CrO8 is +5.
Note:
D-block elements are also known as transition metals and they form coordination compounds. Coordination compounds are formed when coordinate bonds are formed between the metal atom or ion and ligands. In the above case, the central atom or ion is chromium and the ligands are oxygen. So, oxygen donates its lone pair to the low lying vacant orbitals of the metal.
