Question
Question: What is the structure of diborane: A. four \(2c - 2e\) bonds four \(3c - 2e\)bonds B. two \(2c -...
What is the structure of diborane:
A. four 2c−2e bonds four 3c−2ebonds
B. two 2c−2e bonds and two 3c−2e bonds
C. two 2c−2e bonds and four 3c−2ebonds
D. four 2c−2e bonds and two 3c−2ebonds
Solution
Diborane consists of boron and hydrogen elements with the molecular formula B2H6 . It is a colourless gas with a sweet odour.
Complete step by step answer: In Diborane, four terminal hydrogens and bridging hydrogens between the borons are present.
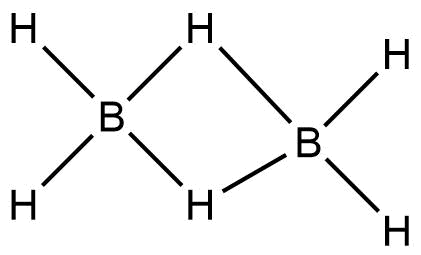
The bonds between the terminal hydrogens and boron is 2−Center,2−electron covalent bond.
Whereas, the bonds between boron and the bridging hydrogens are different. Each Boron atom uses its two electrons in bonding with the terminal hydrogen. The remaining one valence electron participates in bridging. The Boron and bridging hydrogen provide one electron each and form aB2H2 ring, held by four electrons forming two 3−Centre−2 electron
So, the correct answer is “Option D”.
Additional Information: The length of the B (terminal) bond is 1.19 A, whereas the length of the B−H(Bridges) Bond is 1.33A. Due to difference in bond length, bond strength is also different. The B−H(Bridge) bond is relatively weaker than the B−H(terminal) bond.
Note: This type of bond is known as "Banana bond". Gallium also forms a similar compound Ga2H6 , known as "digauane".
