Question
Question: What is the structure of an atom?...
What is the structure of an atom?
Solution
Atoms have three subatomic particles which include electrons, protons, and neutrons. Electrons are negatively charged species while protons are positively charged species. Neutrons have no charge.
Complete answer:
In the center of the atom, the nucleus is present which contains protons and neutrons. Protons are positively charged species and neutrons are neutral species (have no charge). Rutherford’s α-particle scattering experiment on thin gold foil discovered the atomic nucleus. Electrons are revolving around the nucleus in a definite orbit (shells or energy levels). Electrons are negatively charged species. This is illustrated in the following diagram:
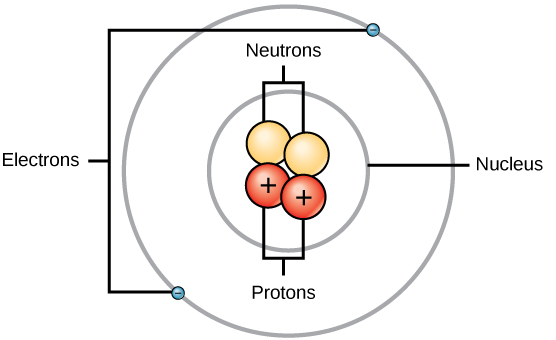
The increasing order of the mass of these subatomic particles are givens as electron<proton<neutron
The presence of protons and neutrons in the nucleus makes it massive. For the electrical neutrality of an atom, the number of electrons is equal to the number of protons. Thus, the atomic number of the atom is equal to the number of protons or the number of electrons. While mass number describes the sum of protons and neutrons. In the formation of cation, electron loss takes place and thus the number of electrons is less than the number of protons. In the formation of anion, a gain of electrons takes place and thus the number of electrons is greater than the number of protons.
Note:
It is important to note that the nucleus is present at the center of the atom. The atomic nucleus contains protons and neutrons which makes it massive and positively charged. Electrons are revolving around the nucleus in definite energy levels.
