Question
Question: What is the structural formulae of butanoic acid?...
What is the structural formulae of butanoic acid?
Solution
In IUPAC naming –but is given as the prefix for compounds which has four carbon atoms in them. The naming of carboxylic acid group is done by giving a suffix as –oic acid.
Complete step-by-step answer: In the question it is asked that we should predict the structure of butanoic acid.
So butanoic acid is a carboxylic acid of the hydrocarbon butane. We are very well-versed with the IUPAC nomenclature of the organic compounds. But for the sake of solving the problems lets brush up our memory.
In the question the IUPAC name is given and we have to find the structure. Now the part we are going to discuss first is to name a compound if a structure is given. We have to memorize some suffix and prefix given for the compounds so that it will be easy for us to solve the organic problems.
First of all let’s see the basics i.e. what will be the name given for compounds having different number of carbon atom.
If in a compound:
1 carbon present prefix used is –meth
2 carbons- eth
3 carbons- prop
4 carbons-but
5 carbons- pent
6 carbons- hex etc.
Now for the functional groups like carboxylic acid is present we will replace the suffix –ane of the main chain with the suffix –oic acid.
For ketone it is –one, aldehyde its –al, for alcohol the suffix used is –ol etc.
If we are provide with a structure and have to give the IUPAC name, first identify the longest carbon chain which serves as the parent chain. Then find the different functional groups and substituents attached to the carbon chain. Then give the number in such a way that on the summation f the number we will get the least number and the structure with least value will be the right way of numbered structure.
Here the name is given hence lets decode the IUPAC name with the aid of the basics discussed above and find the correct structure.
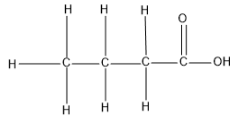
Note: There can only be two carboxylic acid groups in a molecule since they will be always attached only at the end part of the molecule and if two carboxylic acid groups are present, then we should represent its number in the suffix as –enedioic acid.
