Question
Question: What is the structural formula of cyclobutane?...
What is the structural formula of cyclobutane?
Solution
From the name of the compound, we can infer that
- It has a cyclic structure
- It has four carbon atoms
- It does not have any multiple bonds
Complete answer:
We know that cyclobutane is a type of cycloalkane. A cycloalkane is a monocyclic saturated hydrocarbon. It does not contain any atoms or elements except hydrogen and carbon.
Since we know that it has a saturated monocyclic structure and has four carbon atoms, its Lewis structure will be as follows
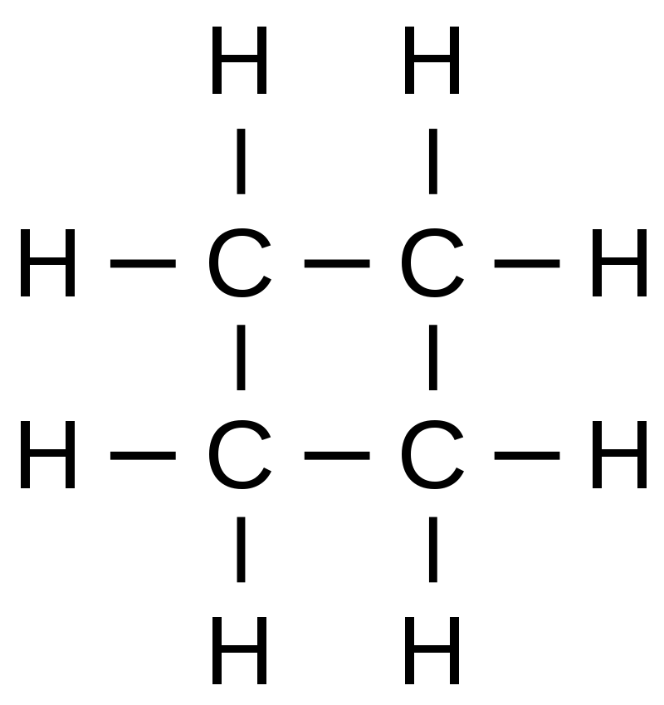
So, from the structure, we can see that it has 8 hydrogen atoms and hence structural formula of cyclobutane will be (CH2)4 or C4H8.
Additional Information:
Some properties of cyclobutane are
- It is prepared by dimerization of alkenes in UV-light. It can also be prepared by dehalogenation of 1,4-dihalobutene in the presence of reducing metals.
- It is a colorless gas but can be liquified for commercial purposes.
- It has a molar mass of 56.107 g/mol.
- Its boiling point is 285.6K and its melting point is 182K.
- Complex derivatives of cyclobutane are of much importance in the fields of biotechnology and biology.
- It does not have much biological or commercial significance.
- Carbon-carbon bonds in cyclobutane have lower bond energy and have significantly strained bond angles. This is why it is unstable at a temperature above 500∘C.
Note:
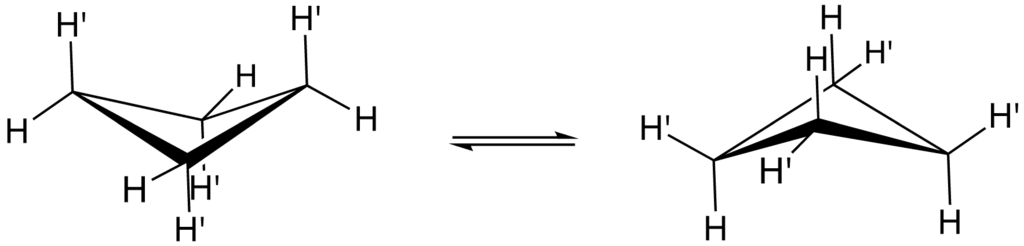
It should be noted that the four carbon atoms in the cyclobutane compound are not coplanar. A puckered or a folded conformation is adopted by the cyclobutane ring. The angle between the plane formed by the three carbon atoms and the fourth carbon atom is 25∘.