Question
Question: What is the stoichiometric coefficient of SO$_2$ in the following balance reaction? MnO$_4^-$ (aq) ...
What is the stoichiometric coefficient of SO2 in the following balance reaction?
MnO4− (aq) + SO2(g) ⟶ Mn2+(aq) + HSO4−(aq)
(in acidic solution)
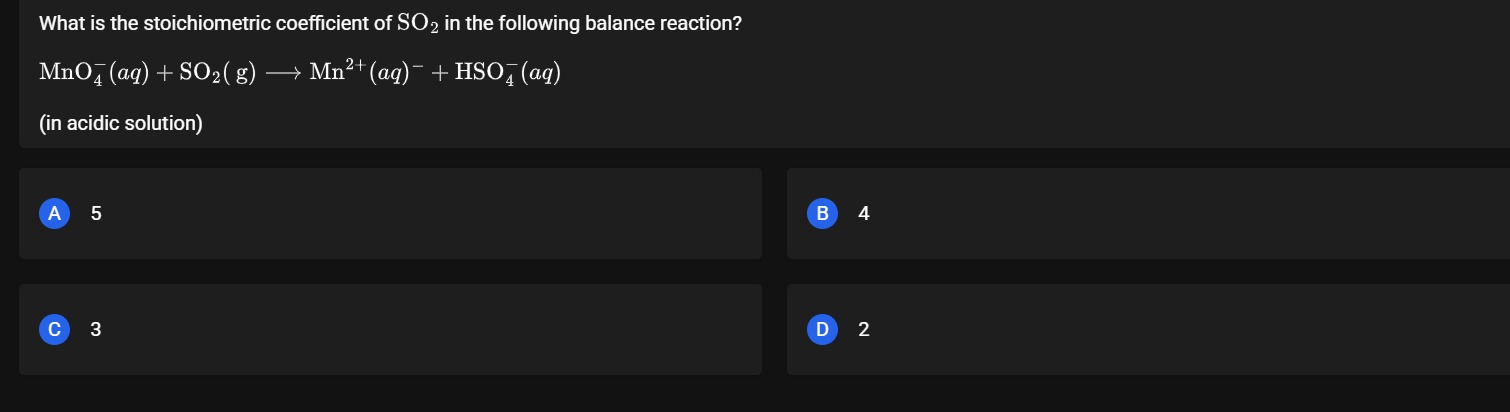
5
4
3
2
The stoichiometric coefficient of SO2 in the balanced reaction is 5.
Solution
To balance the given redox reaction in acidic solution, we will use the ion-electron method. The unbalanced reaction is:
MnO4− (aq) + SO2(g) ⟶ Mn2+(aq) + HSO4−(aq)
Step 1: Identify the oxidation and reduction half-reactions.
Manganese is reduced from +7 in MnO4− to +2 in Mn2+.
Sulfur is oxidized from +4 in SO2 to +6 in HSO4−.
Reduction half-reaction: MnO4− ⟶ Mn2+
Oxidation half-reaction: SO2 ⟶ HSO4−
Step 2: Balance atoms other than O and H.
Mn and S atoms are already balanced in both half-reactions.
Step 3: Balance O atoms by adding H2O.
Reduction: MnO4− ⟶ Mn2+ + 4H2O (4 O on left, 0 on right ⟹ add 4H2O to right)
Oxidation: SO2 + 2H2O ⟶ HSO4− (2 O on left, 4 O on right ⟹ add 2H2O to left)
Step 4: Balance H atoms by adding H+ (since the solution is acidic).
Reduction: MnO4− + 8H+ ⟶ Mn2+ + 4H2O (0 H on left, 8 H on right ⟹ add 8H+ to left)
Oxidation: SO2 + 2H2O ⟶ HSO4− + 3H+ (4 H on left, 1 H on right ⟹ add 3H+ to right)
Step 5: Balance charge by adding electrons (e−).
Reduction: MnO4− + 8H+ + 5e− ⟶ Mn2+ + 4H2O (Left charge: -1 + 8 = +7; Right charge: +2. Add 5e− to left)
Oxidation: SO2 + 2H2O ⟶ HSO4− + 3H+ + 2e− (Left charge: 0; Right charge: -1 + 3 = +2. Add 2e− to right)
Step 6: Multiply the half-reactions by appropriate integers to equalize the number of electrons transferred.
Multiply the reduction half-reaction by 2 (to gain 10e−).
Multiply the oxidation half-reaction by 5 (to lose 10e−).
2 × (MnO4− + 8H+ + 5e− ⟶ Mn2+ + 4H2O)
⟹ 2MnO4− + 16H+ + 10e− ⟶ 2Mn2+ + 8H2O
5 × (SO2 + 2H2O ⟶ HSO4− + 3H+ + 2e−)
⟹ 5SO2 + 10H2O ⟶ 5HSO4− + 15H+ + 10e−
Step 7: Add the balanced half-reactions and cancel common species on both sides.
(2MnO4− + 16H+ + 10e− ⟶ 2Mn2+ + 8H2O)
- (5SO2 + 10H2O ⟶ 5HSO4− + 15H+ + 10e−)
2MnO4− + 16H+ + 10e− + 5SO2 + 10H2O ⟶ 2Mn2+ + 8H2O + 5HSO4− + 15H+ + 10e−
Cancel 10e− from both sides.
Cancel 15H+ from 16H+ on the left, leaving 1H+ on the left.
Cancel 8H2O from 10H2O on the left, leaving 2H2O on the left.
The balanced equation is:
2MnO4− (aq) + 5SO2(g) + H+ (aq) + 2H2O (l) ⟶ 2Mn2+(aq) + 5HSO4−(aq)
Therefore, the stoichiometric coefficient of SO2 in the balanced reaction is 5.
