Question
Question: What is the stereo chemical result of \({{S}_{N}}^{1}\text{ and }{{S}_{N}}^{2}\text{ }\) reactions?...
What is the stereo chemical result of SN1 and SN2 reactions?
Solution
SN1 Reactions are unimolecular reactions, they have a stepwise mechanism whereas SN2 reaction is a bimolecular reaction, and it is a single-step reaction.
Complete step by step solution:
SN1 and SN2 Reaction both are nucleophilic substitution reaction, it involves the attack of a positively charged or a partially positively charged atom or group by a nucleophile. Nucleophiles are the species which are rich in electrons; they can donate an electron pair.
SN1 Reaction follows the first-order kinetics and it is a two-step reaction. The rate of the reaction depends upon the concentration of the substrate. A carbocation is formed as an intermediate in the SN1 reaction after the removal of the leaving group. SN1 Reaction gives the mixture of the product with the retention and inversion in the configuration. This leads to racemization.
Mechanism of SN1 reaction is shown below:
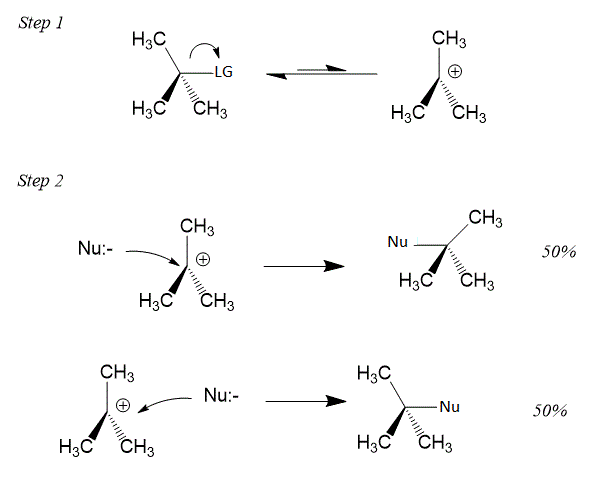
SN2 Reaction follows the second-order kinetics and it is a single step reaction. The rate of the reaction depends upon the concentration of the substrate and nucleophile. There is a formation of a single transition state in SN2 reaction.
SN2 Reaction leads to a back-side attack, which leads to the inversion of the stereochemistry of the carbon atom, here a complete inversion of the configuration takes place.
Mechanism of SN2 reaction is mentioned below:
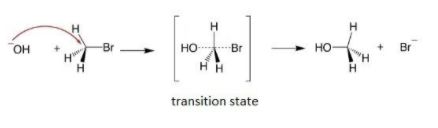
Note: For both SN1 and SN2 reaction higher will be temperature more elimination product we get. The more elimination products we get, the substitution product will be less because the amount of the reactant is limited. This is because the activation energy for a particular reaction is higher for the elimination reaction than the substitution reaction for the same reaction.
