Question
Question: What is the state of hybridization of carbon in (a) \[C{O_3}^{ - 2}\](b) diamond (c) graphite?...
What is the state of hybridization of carbon in (a) CO3−2(b) diamond (c) graphite?
Solution
Hint : First we have to draw the hybridization structure of given compounds. As we know the hybridization of a compound depends upon the number of σbonds. So we have to count the number of σ bonds which are formed by C.
Complete step by step solution :
In a compound, if the number of bonds is 4 then the Hybridisation will be sp3. If the number of σ bond exists as 3 then hybridization will be sp2 and if the number of σ bonds is 2 then the Hybridisation will be sp.
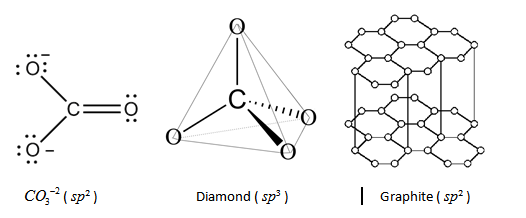
The state of hybridisation of Carbon in CO3−2 is sp2, hybridization in diamond is sp3 and hybridization in graphite is sp2 respectively.
Note : We must know that diamond and graphite both are the allotropes of carbon and so they have identical chemical properties. In graphite, each atom shares electrons with 3 different carbon atoms, whereas in diamond, each atom here shares the electrons with 4 other carbon atoms. Diamond is very strong in comparison to graphite as graphite exhibits a brittle and soft state. The sheets of carbon become bonded with weak covalent bonds during a tetrahedral structure. The other sheets of carbon become bonded by weak intermolecular forces. Due to the weak intermolecular forces, the layers of graphite can slide upon each other and make the substance very weak in comparison with diamonds. Also, diamond is difficult to break due to its giant covalent lattice and its many strong covalent bonds.
