Question
Question: What is the special feature of the structure of ethyne?...
What is the special feature of the structure of ethyne?
Solution
“eth” indicates two carbon atoms are present and “yne” indicates that a triple bond is present. Ethyne is a compound containing two carbon atoms connected with a triple bond between them and it is commonly known as Acetylene.
Complete answer:
According to the IUPAC nomenclature, “eth” signifies two carbon compounds and “yne” signifies a triple bond between the two carbon compounds.
Hence, the general formula for alkyne is CnH2n−2
Where n is the number of carbon atoms.
The chemical formula for ethyne is (C2H2)
The structure is ethyne is as follows:
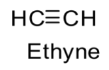
Special feature of the structure of ethyne is the presence of a triple bond.
The structure of ethyne is unstable when it is in its pure form as it is an unsaturated hydrocarbon. The bond angle between the carbon atom i.e. where triple bond is present is almost 180o . Also, the bond length of the C−H bond is around 106 picometers and the bond length of the C−C bond is around 120.3 picometers.
Therefore, in the case of ethyne, every carbon atom is connected with a hydrogen atom by a single bond at one end and with another carbon atom with a triple bond at another end. Ethyne is a symmetrical compound.
Note:
Remember, the presence of a triple bond is a special feature of the structure of ethyne. Some chemical and physical properties of ethyne molecules are that under the standard condition of temperature and pressure (STP) behaves like a colorless gas. It slightly dissolves in water. It does not have any distinct color. Ethyne is a gas and its melting point is −80.8oC.
