Question
Question: What is the shape of \(\text{ Cl}{{\text{F}}_{\text{3 }}}\) molecule? (A) Trigonal planar (B) Tr...
What is the shape of ClF3 molecule?
(A) Trigonal planar
(B) Trigonal pyramidal
(C) T-shaped
(D) Tetrahedral
Solution
The valence shell electron pair repulsion (VSEPR) theory is used to determine the shape of molecules. According to VSEPR theory the lone pair-lone pair has the maximum repulsion followed by the bond pair-lone pair and bond pair –bond pair. In ClF3 two lone pairs are placed in axial plane which minimises the repulsion and stabilises the molecule.
Complete Solution :
In ClF3 , the chlorine is a central atom. The electronic configuration of chlorine atom is as below:
Cl = 1s2 2s2 2p6 3s2 3p5
Chlorine atom has seven valence electrons. In ClF3 molecule, the central chlorine atom is surrounded by three fluorine atoms. Each fluorine atom shares a one electron with chlorine atom and results in three covalent Cl−F bonds.
The ClF3 molecule has 7 valence electrons from chlorine and three from Fluorine. Thus there are a total 10 electrons around the central chlorine atom.
Now let’s determine the electron pair in ClF3 molecule. Divide total number of electrons by 2.
Electron pair = 210= 5 e−
There are a total of 5 electron pairs in ClF3 molecule. Let’s calculate the number of lone pairs in the ClF3 molecule. The lone pair of electrons can be determined by subtraction of the number of bond pairs from the electron pair. As follows:
Lone pair = Electron pair−Lone pair = 5−3 = 2
Now we know that ClF3 molecule has three bonding pairs and two lone pairs. Now according to VSEPR theory the lone pair –lone pair experience the maximum repulsion which can be reduced by placing two lone pairs in the axial plane. The shape of ClF3 molecule is as shown below:
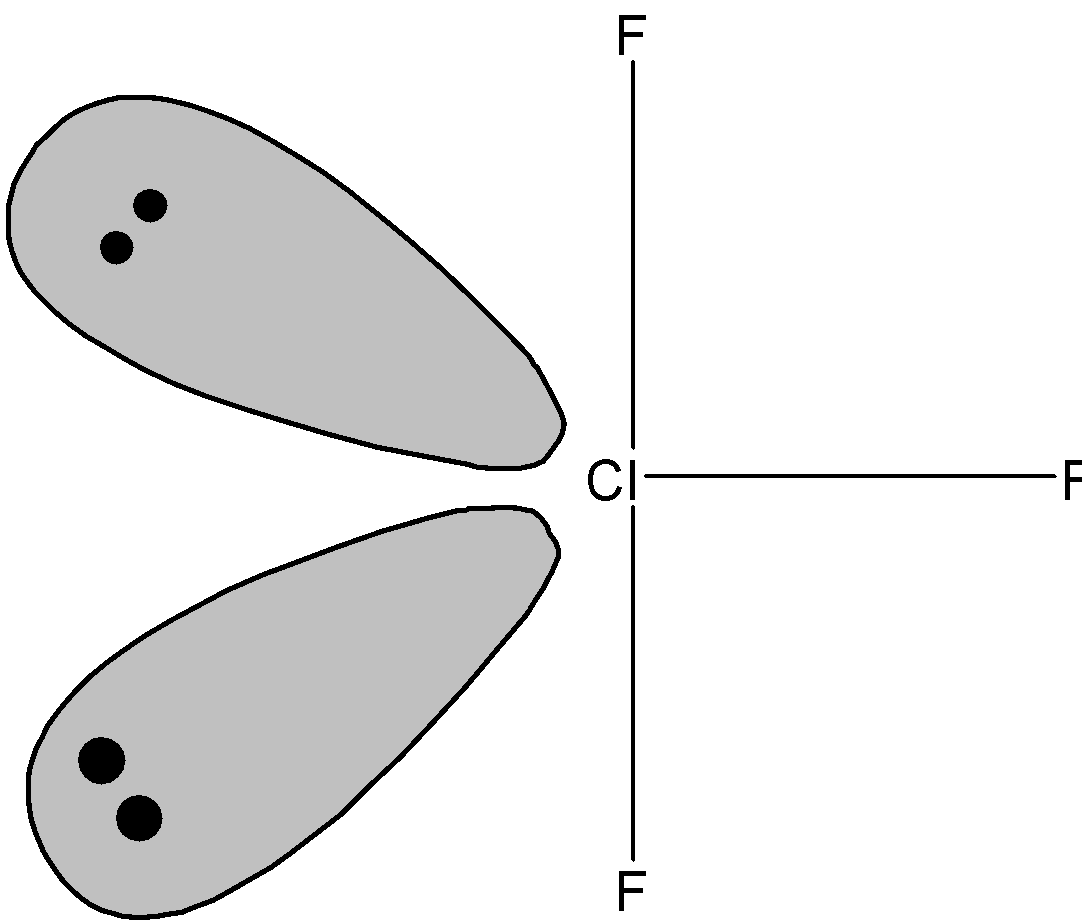
Therefore, ClF3 molecule shows T-shaped geometry.
So, the correct answer is “Option C”.
Note: From local observation, we can say that ClF3 is a trigonal planar molecule. But, one should always consider the lone pair on the central atom. The number of lone pairs can be determined by a formula.
No.of LP = BP + 21[Group attached−Valency ± Charge]⇒No.of LP = 3 + 21[5−7]∴No.of LP in ClF3= 3 −1 = 2 L.P.
