Question
Question: What is the shape of ethene molecule? A) Planar B) Tetrahedral C) Bipyramidal D) None of the...
What is the shape of ethene molecule?
A) Planar
B) Tetrahedral
C) Bipyramidal
D) None of these
Solution
It is easy to identify the shape of a molecule if we know the hybridization of that molecule. Alkanes, Alkenes and Alkynes are the hydrocarbons that contain bonds between carbon and hydrogen atoms.
Complete step by step answer:
We have to know that the ethene has a molecular formula C2H4. It has a double bond and therefore belongs to alkene.
We can write the general formula of Alkene as: CnH2n
We need to know that the electronic configuration of C in ground state: 1s22s22p2
Excitation of electron from s orbital to vacant p orbital so,
We must know that the electronic configuration of C in excited state: 1s22s12p3
Therefore, Hybridization of Ethene= sp2 one electron will be unpaired that is an unhybridized orbital and 3 sp2orbitals.
We need to remember that the ethene has geometry is Planar
We can draw the structure of ethane molecule as,
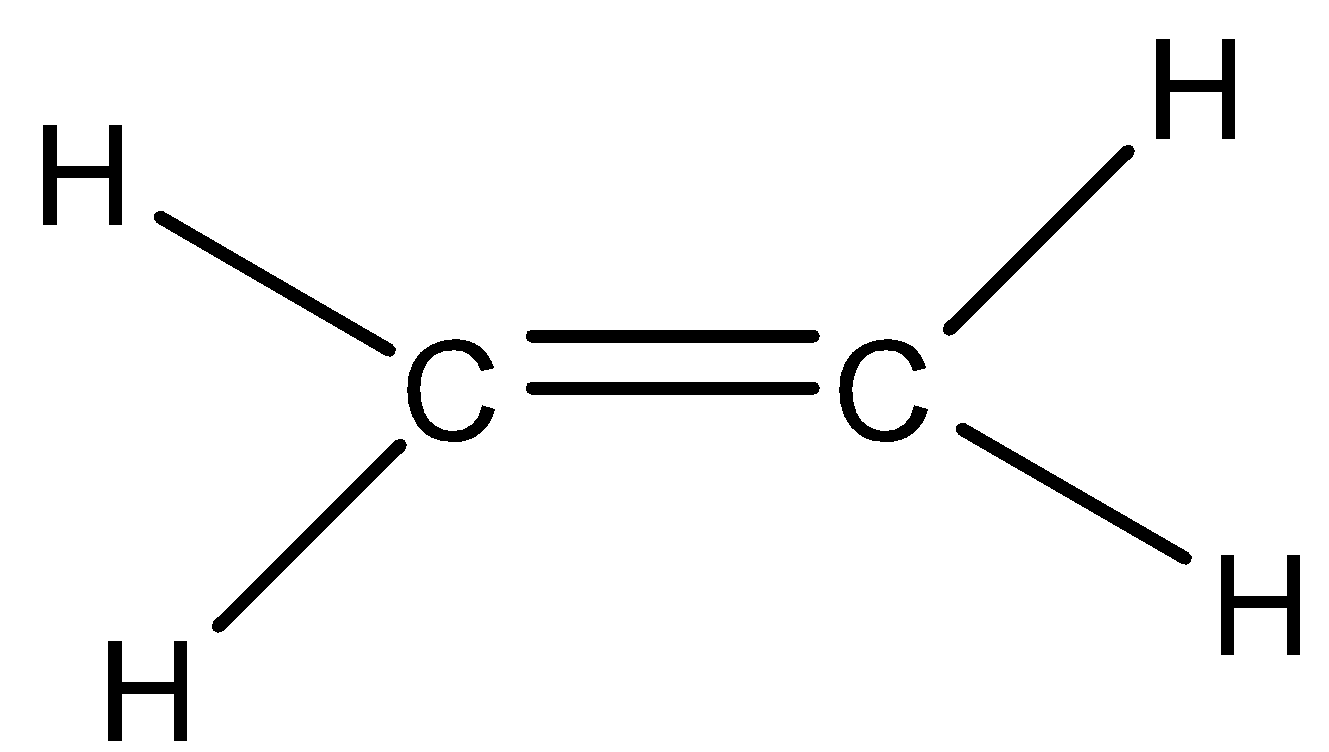
Option A) this is a correct option as the molecule has a planar shape as it has sp2 hybridization.
Option B) This is an incorrect option as tetrahedral geometry is for sp3 hybridization.
Option C) this is an incorrect option as bipyramidal geometry has a hybridization of sp3d.
Option D) this is an incorrect option as we got a correct option as ethene has planar shape.
Hence, the correct answer is, ‘Option A’.
Note: We need to remember that the ethane has molecular formula C2H6; Ethene has a molecular formula C2H4 and ethylene has molecular formula C2H2 having slightly sweet smell. We need to know that the ethylene is a nonpolar molecule and soluble in non-polar solvents.
