Question
Question: What is the shape of \[C{H_4}O\]?...
What is the shape of CH4O?
Solution
Molecules are the combination of atoms or chemical elements. The atoms are attached through bonding. Methanol is a molecule with two geometric centres; one is oxygen and the other is carbon. The geometry of the molecule can be determined from the valence electrons and the hybridisation.
Complete answer:
Given molecule is methanol. Due to the presence of hydroxyl group, it comes under alcohols. The IUPAC nomenclature of alcohols has the suffix of -al. The molecular formula of methanol is CH4O. It has one carbon atom, one oxygen atom and four hydrogen atoms. Thus, methanol has two geometric centres, one is oxygen and the other is carbon.
Carbon is an element with atomic number 6 and the valence electrons are 4.
Oxygen is an element with atomic number 8 and the valence electrons are 6.
Hydrogen is an element with atomic number 1 and the valence electrons from four hydrogen atoms are4.
The total number of valence electrons from all the atoms in methanol are 14. These 14 electrons are distributed between the atoms to form a covalent bond. The total electrons involved in bonding are 10. The remaining four electrons exist as lone pairs on oxygen atoms.
The structure of methanol will be as follows:
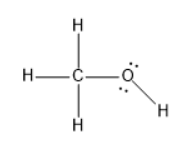
The carbon is tetrahedral geometry and the molecular geometry is also tetrahedral.
The oxygen has tetrahedral electron geometry and the molecular geometry is bent.
Note:
All the atoms in methanol are single bonded and have sp3 hybridization. Thus, the electron geometry will be a tetrahedron. But, the two lone pairs on an oxygen atom makes the oxygen atom to be bent in molecular geometry.
