Question
Question: What is the role of tartaric acid in baking powder?...
What is the role of tartaric acid in baking powder?
Solution
Neutralization reaction is one of the most important reactions which is given by different acids and bases. When equal amounts of acid and base are mixed together, it will result in formation of salt and water.
Complete Step By Step Answer:
Tartaric acid is a natural component which is obtained from natural resources like grapes, tomatoes and bananas. Tartaric acid acts as a slight astringent. Structure of tartaric acid contains two carboxylic acid groups, which is why it is an example of dibasic acid.
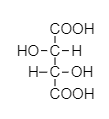
Baking soda is chemically a basic compound, it is a bicarbonate of sodium element which is commonly known as sodium bicarbonate. Molecular formula of baking soda is NaHCO3 .
When we add baking soda and tartaric acid together due to differences in their nature, they undergo a neutralization chemical reaction. Neutralization reactions occur because baking soda is a base and tartaric acid is an acid.
When baking soda is added in food items it will release sodium carbonate at elevated temperature which will cause the bitterness of taste.
2NaHCO3→Na2CO3+H2O+CO2
So, when we previously add tartaric acid, the released sodium carbonate reacts with tartaric acid to form salt of sodium tartrate and that is how bitter taste of food item is prevented.
So final reaction which occur on adding tartaric acid in baking powder is:
NaHCO3+C4H6O6→Na2C4H4O6+H2O+CO2
⇒ The main role of tartaric acid in baking powder is to avoid the bitter taste by neutralizing the sodium carbonate produced by baking powder.
Note:
This property of generating carbon dioxide gas during reaction both of these constituents are highly used in baking industries to increase the puffiness of baked food items due to entrapment of carbon dioxide gas. Tartaric acid is also used as a main constituent in wine preparation.
