Question
Question: What is the role of pyridine in the acylation of amines?...
What is the role of pyridine in the acylation of amines?
Solution
Hint : in the given question we should know about the chemical reaction of the acylation of amines. When the acyl group is added to a molecule, that type of chemical reaction is called an acylation reaction. Amines are the organic compounds which contain a nitrogen atom with a lone pair of electrons. Acylation of amines is the reaction of amines with the acetyl chloride.
Complete Step By Step Answer:
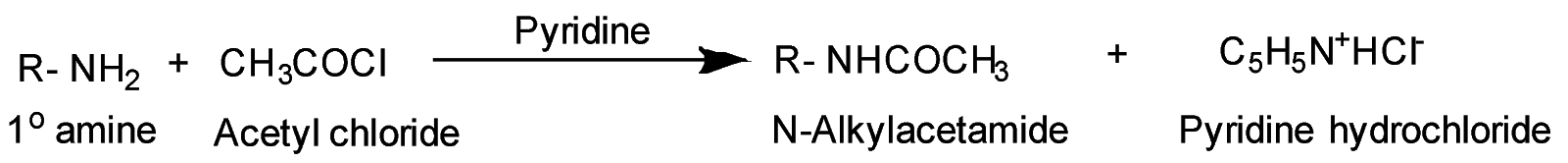
Acylation of amines is the reaction between the amines ( −NH2 group) and the acetyl group ( CH3CO− ) that comes from either acetyl halide or acetic anhydride. This reaction takes place in the presence of pyridine ( C5H5N ) which acts as a strong base during the reaction. During substitution reaction of acylation of ( −NH2 ) group with acetyl group followed by hydrolysis of substituted amide with substituted amine, the activating effect of −NH2 group can be controlled. Pyridine is used to remove the side product formed in the acylation reaction i.e. HCl from the reaction mixture. It acts as an acceptor for the acid byproduct formed in the reaction.
Pyridine also acts as a nucleophile for the carbonyl group due to its lone pair of electrons on nitrogen atoms which can be delocalized in its ring. It acts as a catalyst and is often used in the acylation reactions.
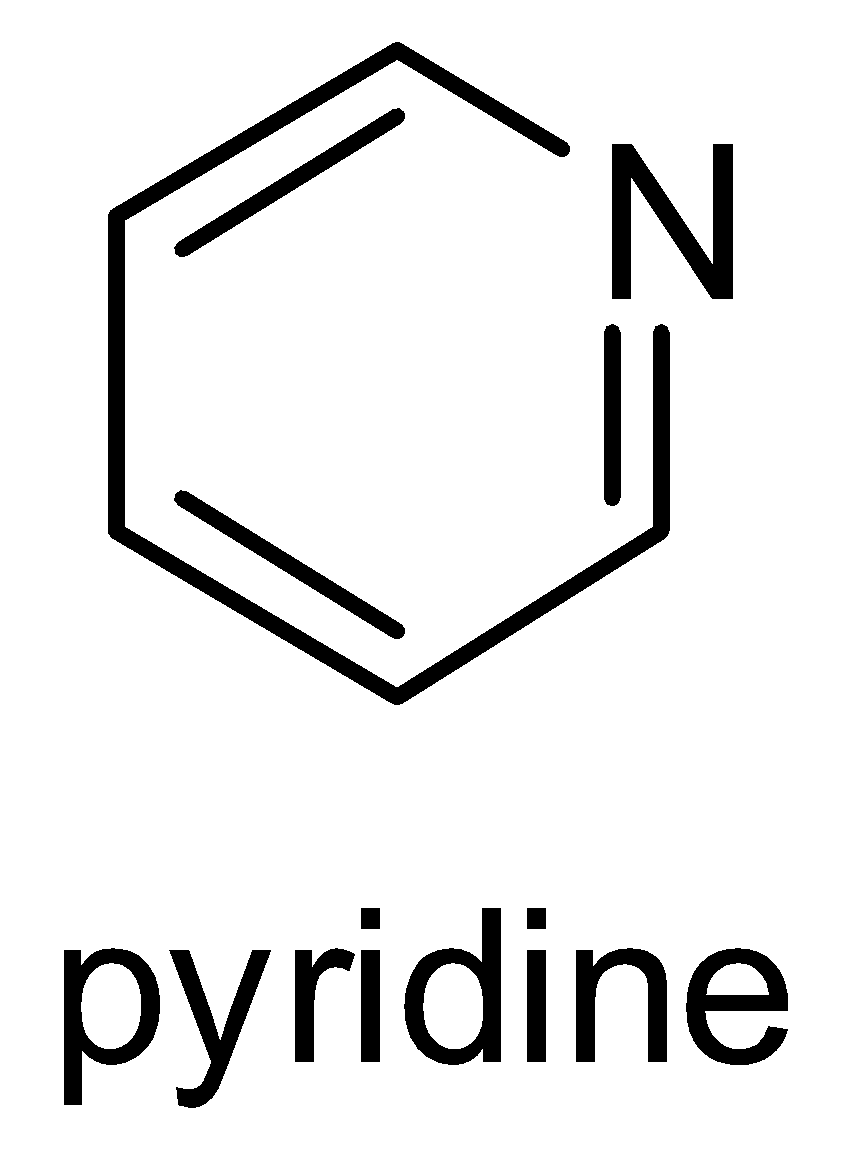
Structure of Pyridine-
Example- Chemical Reaction between primary amine and acetyl chloride
Note :
It needs to be remembered that pyridine is a basic heterocyclic organic compound that acts as a base as well as nucleophile. It has a lone pair of electrons on nitrogen atoms which get delocalized in the ring. Structure of pyridine and its molecular formula ( C5H5N ) should be remembered. Pyridine is a water- miscible liquid with a distinctive and unpleasant fish like smell. It is a highly flammable and weakly alkaline liquid.
