Question
Question: What is the radius of Sodium atom if it crystallizes in BCC unit cell edge length of \( 400pm \)?...
What is the radius of Sodium atom if it crystallizes in BCC unit cell edge length of 400pm?
Solution
The unit cell is the smallest repeating unit of a crystal lattice. There are different types of crystal lattices, out of which one is body-centered cubic BCC which can be seen in the below image:
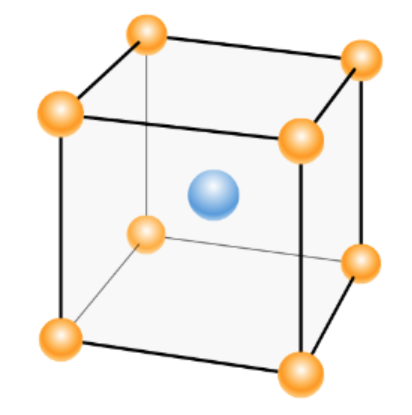
When any atoms are arranged in a body-centered cubic cell, the radius will be determined by substituting the edge length in the below formula.
r=43×a
r is radius of a body centered cubic unit cell
a is edge length.
Complete answer:
Given that the sodium atom is crystallized in a body-centered cubic cell, with the edge length of 400pm.
We know the radius formula in BCC as
r=43×a
Substituting this edge length in the above formula,
r=43×400
On simplification, we get the radius as
r=173.2pm
**Thus, the radius of the sodium atom if it crystallizes in BCC unit cell edge length of 400pm is 173.2pm .
Additional information:
• Crystallography is a branch that deals mainly with crystal structures. According to this concept, the crystal structure is an order of the arrangement of atoms, ions, or molecules.
• The crystal lattice is also known as crystal structure which is nothing but the arrangement of atoms, ions, or molecules in the form of a space lattice.
• They are different types of crystal structures like body-centered cubic (BCC), face-centered cubic (FCC), and hexagonal cubic packing (HCP).
Note:
• While calculating the radius of a crystal lattice, the edge length is usually taken in the units of picometers as these measurements are very minute.
• 1 picometer is equal to 10−12 meters. Picometers can be simply represented as pm.
• If the edge length is given in meters or angstroms, conversion should be made. Where one angstrom is equal to 100 picometers.
