Question
Question: What is the product P in the following reaction? \(C{{H}_{3}}CHO+HCHO\left( excess \right)\xrighta...
What is the product P in the following reaction?
CH3CHO+HCHO(excess)NaOH(P)+HCOONa
(A) 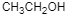
(B) 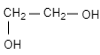
(C) 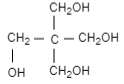
(D) 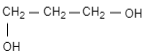
Solution
Cannizzaro reaction is defined as a chemical reaction that shows disproportionation of two molecules of aldehyde and results in the formation of alcohol and carboxylic acid. In this reaction, one aldehyde is oxidised to form acid and the other is reduced to form alcohol.
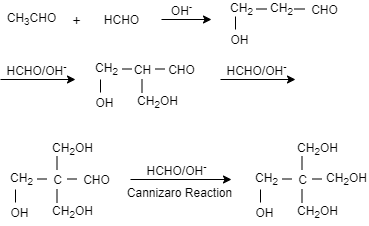
Complete step-by-step answer: When an acetyl aldehyde is reacted with methanol in presence of base that is sodium hydroxide, it results in the formation of 1−hydroxypropanal. When again methanal is added in presence of sodium hydroxide it results in the formation of 3− hydroxy −2− (hydroxymethyl)propanal. Then again methanol and sodium hydroxide are added which result in the formation of 3−hydroxyl −2,2− bis(hydroxymethyl)propanal. When again methanol and sodium hydroxide is added it results in the formation of pentaerythritol.
Additional information: Let us see the mechanism of Cannizzaro reaction:
Step 1:Sodium hydroxide acts as a nucleophile that attacks on the carbonyl group of the aldehyde. Disproportionation reaction takes place and two negative charges arise in an anion.
Step 2:An intermediate is formed which acts as a hydride reducing agent. The intermediate releases hydride anion because of its unstable nature. This hydride attacks on another aldehyde and the charged anion is converted into carboxylate anion and the aldehyde gets converted into alkoxide anion.
Step 3:In this step, protonation takes place to alkoxide anion that results in the formation of an alcohol.
Therefore, the correct option is (C).
Note: In this question, cross Cannizzaro reaction takes place. When a formaldehyde is treated with non enolizable aldehyde in presence of base, aldehyde is converted into alcohol and formaldehyde is oxidised to form a formic acid.
In this question, the product formed is pentaerythritol and sodium formic acid.
