Question
Question: What is the product of the reaction? 
Solution
Elimination reaction is a type of organic chemical reaction in which the two substituents are removed from a compound or molecule via one step or two steps. The ethoxide ion serves as a base for abstracting protons.
Complete step by step answer:
Elimination reaction leads to removal of molecules from a compound. The leaving groups result in dehydration and dehydrohalogenation. The elimination reaction is of three types, E1 , E2 and E1cb.
-The mechanism of elimination reaction depends on the thermodynamics and the kinetics of a reaction. The mechanism involves abstraction of protons and removal of leaving groups with formation of pi bond.
-In the given compound the bromo group attached to the carbon atom is the leaving group. The reaction takes place by abstraction of the proton present on the beta carbon atom adjacent to the bromo substituted carbon.
-The elimination results in formation of a double bond between the alpha and beta carbon atoms. The product of the reaction can be derived using the mechanism of the elimination reaction.
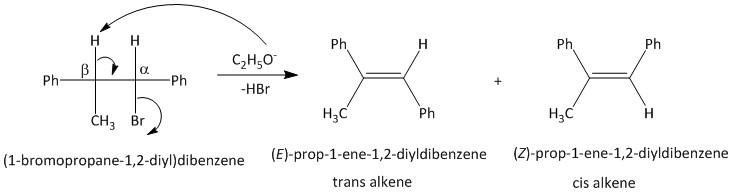
-Theoretically two products (trans and cis) are possible for an elimination reaction from a unsymmetrical alkane. The thermodynamic stability of the products and the mechanism of the reaction will decide the outcome of the product alkene and its configuration.
-In this compound (1 -bromopropane-1,2 -diyl)dibenzene, an E2 type of elimination reaction or an anti elimination will occur to produce a trans alkene.
Note:
E1 elimination is also competitive with the E2 elimination. In E1 elimination a carbanion is formed by abstraction of protons. The relative stability of the carbanion decides the product of the reaction.
