Question
Question: What is the product of the reaction? 
A.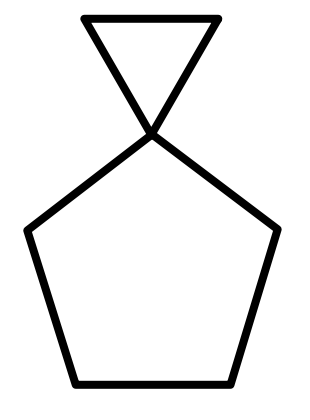
B.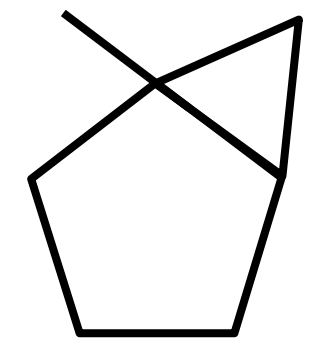
C.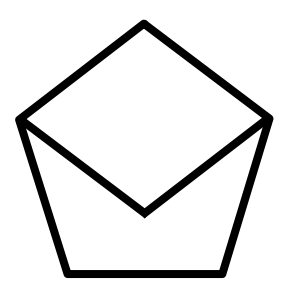
D.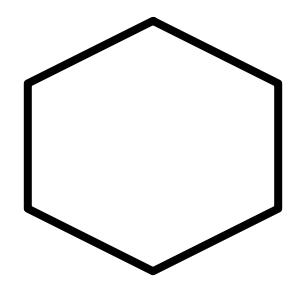
Solution
We know that to prepare iodides by reaction. It is commonly used as a reducing agent and production of acetic acid. CH2I2 is a colorless gas; it has a sharp odor which is dissolved in water. HI is a corrosive acid. Diethyl ether has the ability to lose a proton or take it during chemical reaction.
Complete answer:
The reaction takes place and gives the product of alcohol and iodide. The reaction occurs between two reactants and finally react with each other and give product. Diethyl ether contains ether groups and reacts with diethyl ether. When the reaction takes place, the product is formed of alcohol and iodide. The addition of hydrogen is known as reduction whereas the removal of hydrogen is known as oxidation. The addition of electrons is also known as reduction. The metals generally used for the reduction of unsaturated hydrocarbons to saturated hydrocarbons are platinum, palladium and nickel. In place of Zn/Cu couples in alcohol, the zinc in presence of acid can also be used for the reduction of alkyl halide to form alkane. The compounds having carbon and hydrogen only are known as hydrocarbons. The saturated hydrocarbons are known as alkane and unsaturated hydrocarbons are known as alkene and alkyne. Ether is a volatile, flammable, colorless liquid with a distinctive odor. It belongs to the large functional group of organic compounds called ethers. Its IUPAC name is alkoxy alkane.
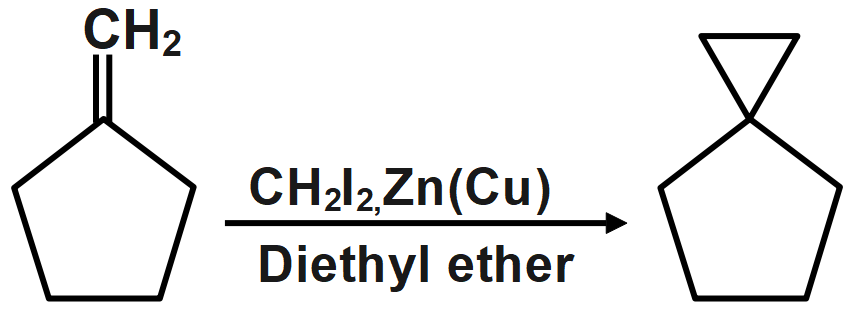
Therefore, the correct answer is option A.
Note:
Remember that the ether undergoes combustion reaction, reacts with oxygen, and forms carbon dioxide and water. It is highly flammable and reacts with halogens like chlorine or bromine to form halogen-substituted ether that undergoes substitution reaction in the absence of sunlight.
