Question
Question: What is the product of the reaction? 
A.
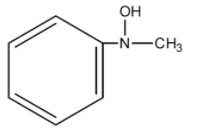
B.
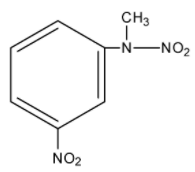
C.
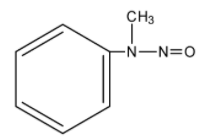
D.
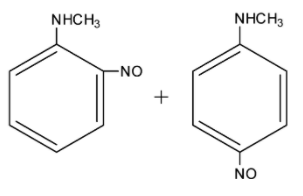
Solution
When nitrous acid and hydrochloric acid mixture is reacted with an amine, then breaking of bonds of R−N takes place and positive charge is formed at nitrogen. It loses hydrogen to get the desired product.
Complete answer:
In order to answer our question, we need to know about some properties of amines. As the nitrogen atom in ammonia molecules is sp3 hybridised and tetrahedral in geometry, similarly ‘N’ atom in all amines also have the same hybridisation, however, they have a pyramidal shape. Out of four hybrid orbitals of nitrogen, three orbitals overlap with hydrogen or carbon atoms of the alkyl group depending upon the composition of amines, whereas the fourth hybrid orbital contains a lone pair of electrons. Repulsion between lone pair and bond pair of electrons suppresses the bond angle which becomes lesser than 109.50 Arylamines have the NH2 group attached directly to the benzene ring. They are named as derivatives of aniline or benzylamine. Aromatic amines are insoluble in water but soluble in ether, alcohol as well as benzene. Primary and secondary amines form intermolecular hydrogen bonding but tertiary amines do not form this due to absence of any hydrogen atom at the nitrogen. One important reaction of amines is the reaction with nitrous acid and hydrochloric acid. Nitrous acid is prepared in situ from a mineral and sodium nitrite. Primary aliphatic amines react with nitrous acid to form aliphatic diazonium salts which being unstable decompose to yield a mixture of nitrogen gas and alcohols. Whereas primary aromatic amines react with nitrous acid at low temperatures to give aromatic diazonium salts. This reaction is called diazotization.
So, the reaction can be expressed as:
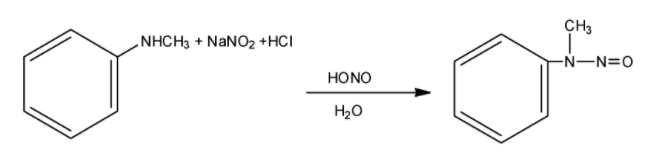
The product we get here is N-Nitroso N-methyl amine, which is a yellow oil.
So, we get our correct answer as option C.
Note:
The diazonium salts are used for synthesis of other organic compounds, preferably aryl compounds. They are very sensitive and can break down if put under ultraviolet light. Many diazonium salts are explosive at high temperatures.
