Question
Question: What is the product of the oxidation of a ketone?...
What is the product of the oxidation of a ketone?
Solution
Hint : In a chemical reaction, oxidation happens when an atom, molecule, or ion loses one or more electrons. The oxidation state of a chemical species increases as it undergoes oxidation. Oxidation does not always require the presence of oxygen.
Complete Step By Step Answer:
A ketone is a functional group that has the structure,
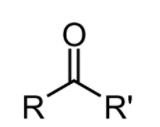
R can be any carbon-based substituent, carbonyl groups, a carbon-oxygen double bond, found in ketone molecules.
A ketone can't be oxidized any further by removing a hydrogen atom. When attempting to oxidize a ketone in this manner, there is no substance.
After that, we're attempting to reduce a ketone. We'll move on to secondary alcohol.
Using the secondary alcohol, we're attempting to achieve a ketone through the Swern oxidation, which necessitates the attachment of a proton to the carbonyl carbon. Since there are no protons attached to the carbonyl carbon in a ketone, this method is impossible to perform.
Baeyer-Villiger oxidation could result in the formation of an ester, but that's a rare reaction.
So, there is no oxidation product for a ketone.
Additional Information:
A difference between an aldehyde and a ketone is that an aldehyde has a hydrogen atom attached to its carbonyl group, making it easier to oxidize. Ketones are more prone to oxidation because they do not have a hydrogen atom bound to the carbonyl group.
Note :
Ketones can be found all over the place in nature. Many carbohydrates are ketones, which are referred to as ketoses collectively. Fructose is the most well-known ketose; it exists as a cyclic hemiketal that hides the ketone functional group. Ketones are used in the synthesis of fatty acids. Acetoacetate is a product of the Krebs cycle, which converts sugars and carbohydrates into energy.
