Question
Question: What is the product of the following reactions? 
(A) 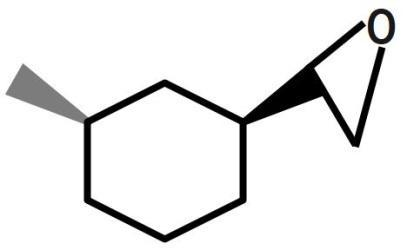
(B) 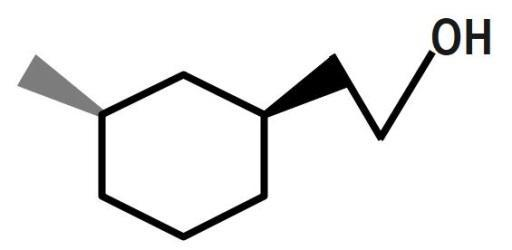
(C) 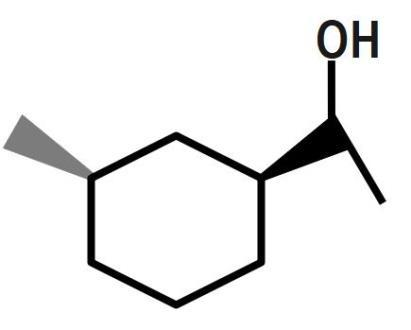
(D) 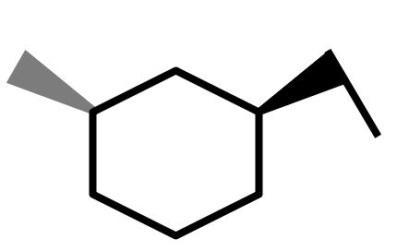
Solution
Hint : We know that the hydroboration oxidation is an anti-markovnikov addition of water across alkene. Also, it is a two-step organic reaction. Hydroboration oxidation reaction is another important chemical reaction of organic chemistry which converts alkene into an alcohol. This reaction gives a more stereo specific and regiochemical alternative to other hydration reactions such as acid-catalyzed addition and oxymercuration reduction reactions.
Complete Step By Step Answer:
Hydroboration oxidation reaction is a two-step reaction in which alkene reacts with BH3, THF (tetrahydrofuran) and hydrogen peroxide in a basic medium to give alcohol. It is also known as HBO reaction. Hydroboration oxidation of symmetrical alkene and unsymmetrical alkene works differently. As the reaction proceeds with unsymmetrical alkene follows Anti-Markovnikov rule. Although as a product we always get alcohol from both alkenes.
It is known to you that hydroboration oxidation is a two-step organic reaction. It is an organic reaction that converts an alkene into an alcohol by the net addition of water across the double bond. THF (Tetrahydrofuran) is used as a solvent. The hydrogen (H) and hydroxyl group (OH) are added in a syn (addition of addendum on the same side of the alkene or alkyne) addition leading to cis stereochemistry. Hydroboration-oxidation is an anti-Markovnikov’s addition of water across an alkene. The −OH group is added to the less substituted position and the -H is in the more substituted position. If there is more than one chiral center in the product, then the product will produce a pair of enantiomers that results from syn addition.
Here, we can see that first, is added to the carbon-carbon double bond in a way that H− atoms get bonded to a carbon that has less number of hydrogen atoms(means in an anti-markovnikov manner). Then, in presence of hydrogen peroxide-an oxidizing agent, the organo-borane compound decomposes and gives an alcohol as shown in the reaction. One molecule can oxidise three molecules of alkene because one B−H bond is responsible for the oxidation of one molecule of alkene.
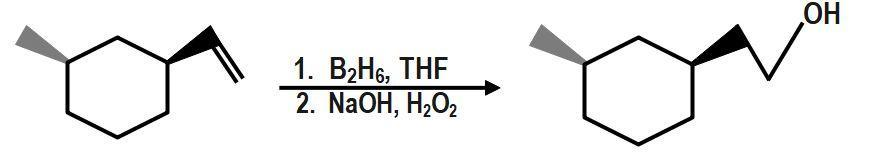
Therefore, the correct answer is option B.
Note :
Remember that it's necessary to understand the chemistry of BH3 before understanding the mechanism of hydroboration oxidation reaction. As boron has one p-orbital empty so BH3 acts as Lewis acid. It exists as a dimer of BH3, it means it exists in the form of B2H62BH3→B2H6 .
