Question
Question: What is the product of the following reaction? 
(A) 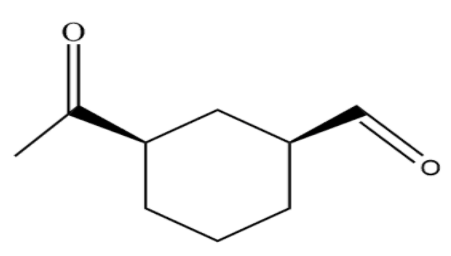
(B) 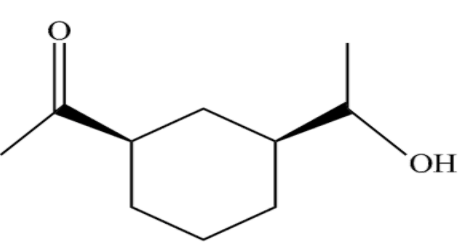
(C) 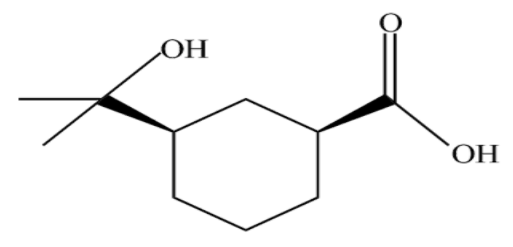
(D) 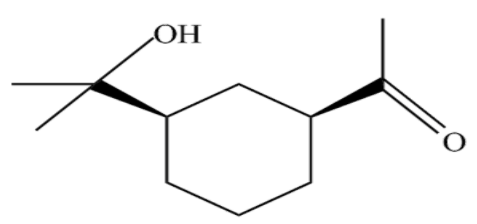
Solution
The reaction involved in this question is known as oxidation of alcohols. This oxidation of alcohol is different from normal oxidation of alcohols because here alcohols are secondary and tertiary. Oxidation of these alcohols can be done through various reactions, Jones reagent, Sarett reagent, Pyridinium Chlorochromate but here we are using Collin’s reagent.
Complete answer:
Collin’s reagent is a complex of chromium (VI) oxide with pyridine in chlorochromate. The molecular formula of Collin’s reagent is C10H10CrN2O3 . This is a red coloured metal complex. This complex is a hygroscopic orange solid. Collin’s reagent is especially used for the controlled oxidation of alcohols to prevent overoxidation so, Collin’s reagent is very useful for acid-sensitive compounds. Collins reagent oxidizes primary alcohols to aldehydes and secondary alcohols to ketones with yield of up to 87−98% .
Now let’s see the reaction of the given compound with Collin’s reagent
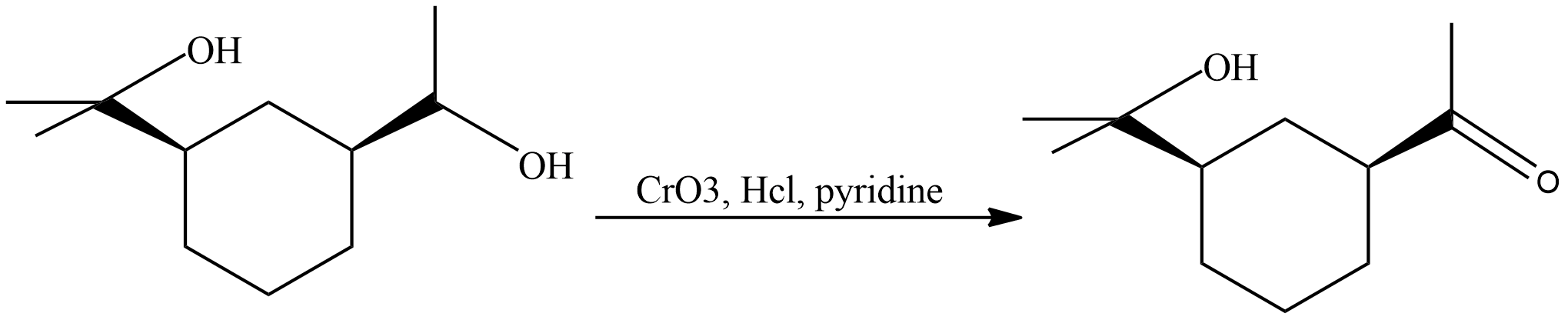
In the above reaction we can see that the secondary alcohol has been converted to the ketone but tertiary alcohol remains the same. Collin’s reagent does not oxidise the tertiary alcohol. Collin’s reagent can be used as an alternative to the Jones reagent and Pyridinium chlorochromate for oxidation of secondary alcohols to ketones.
So, option (D) is the correct answer.
Note:
Tertiary alcohols are the compound in which the Hydroxyl group is attached to the carbon which is attached to three carbons. These compounds do not give oxidation reaction because there is no hydrogen attached to that carbon and in order to set up a double bond, we will have to remove two hydrogen atoms.
