Question
Question: What is the product of the following reaction? 
(A) 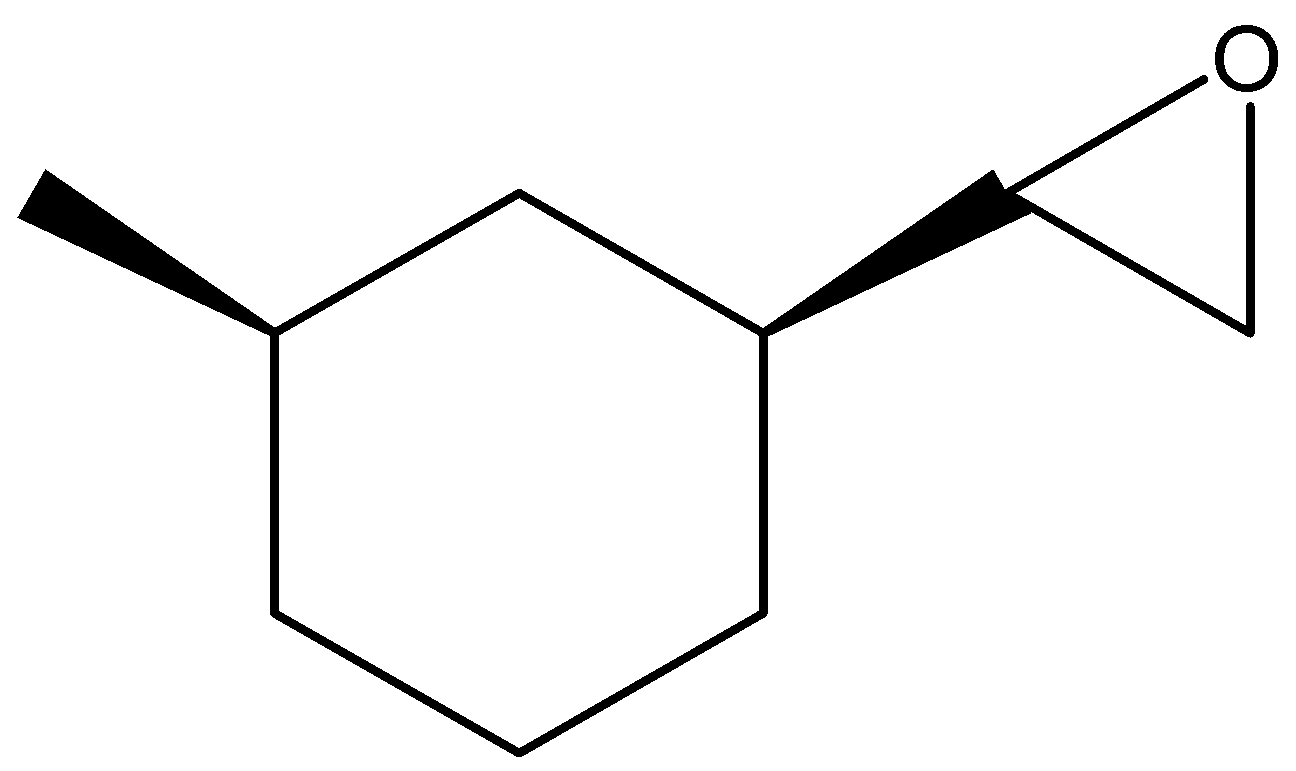
(B) 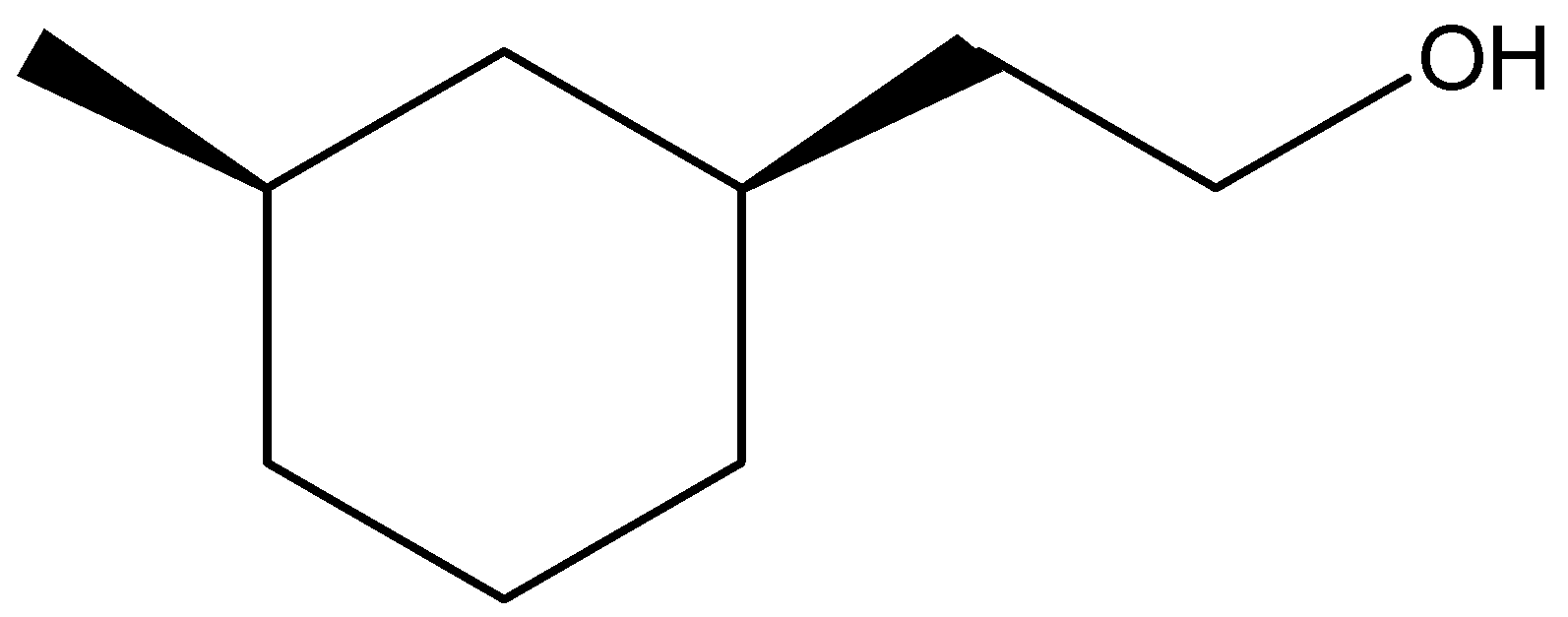
(C) 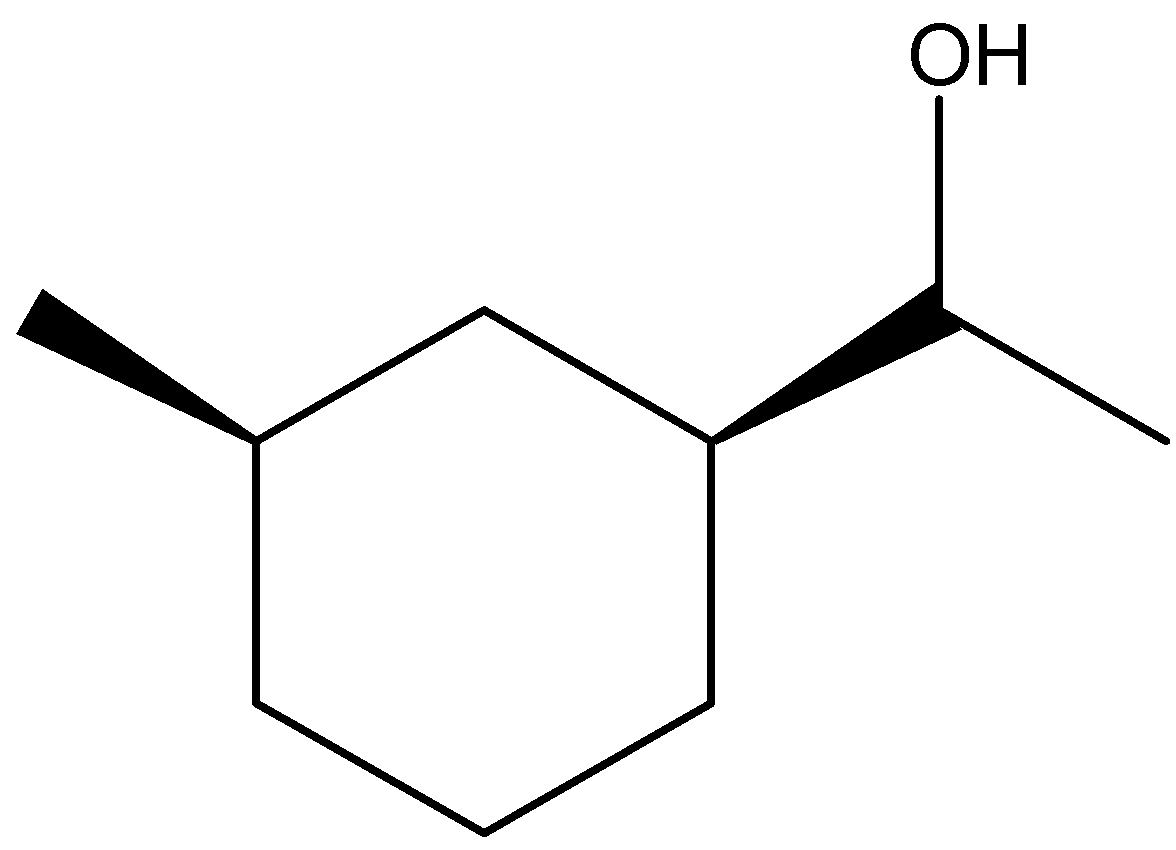
(D) 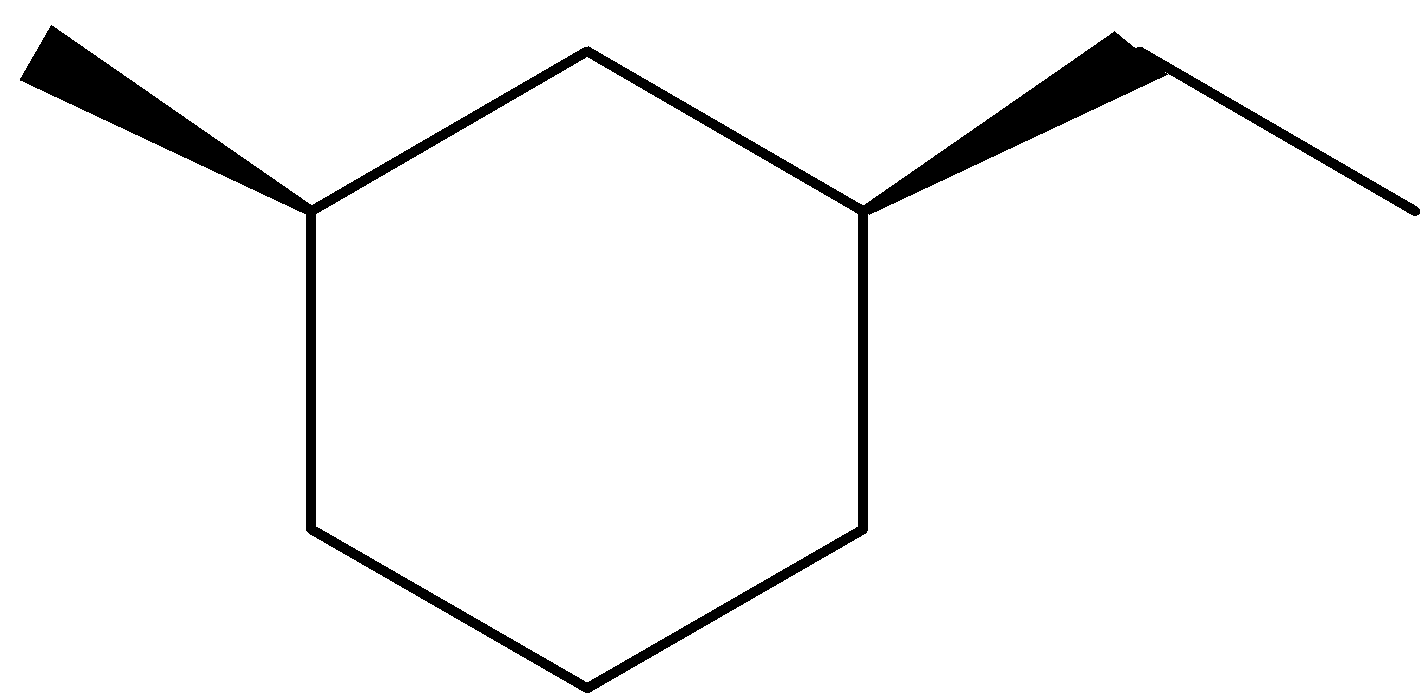
Solution
This is a hydroboration-oxidation reaction. In the first step, a hydroboration reaction will occur across the carbon-carbon double bond. Then, peroxide ions will oxidize the boron and the organic compound.
Complete answer:
We will see the reaction step by step with mechanism.
- B2H6 is known as diborane and in solvent THF ( Tetrahydrofuran), it undergoes hydroboration reaction when allowed to react with an alkene. So, our starting material is also an alkene. So, a hydroboration reaction will occur in which double bonds between the carbon atoms in alkene will get broken and one carbon will form a bond with hydrogen atom while the other carbon atom will form a bond with −BH2. We know that diborane is a dimer of borane. The mechanism of the first step can be given as
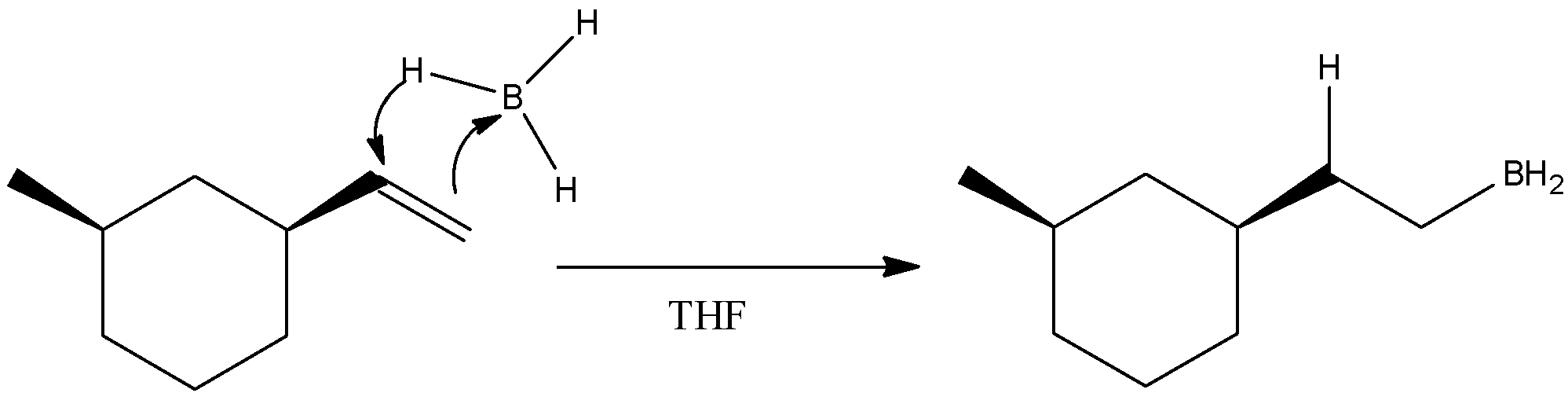
- Then in the second step, the product is allowed to react with NaOH and hydrogen peroxide. The combination of both these reagents is an oxidizing mixture. So, an oxidation reaction will occur here. H2O2 on reaction with NaOH gives peroxide ions.
H2O2+OH−→H2O+HOO−
- These peroxide ions are nucleophilic and attack on the electrophilic boron atom of the organo-boron compound. The reaction is shown with mechanism as below.
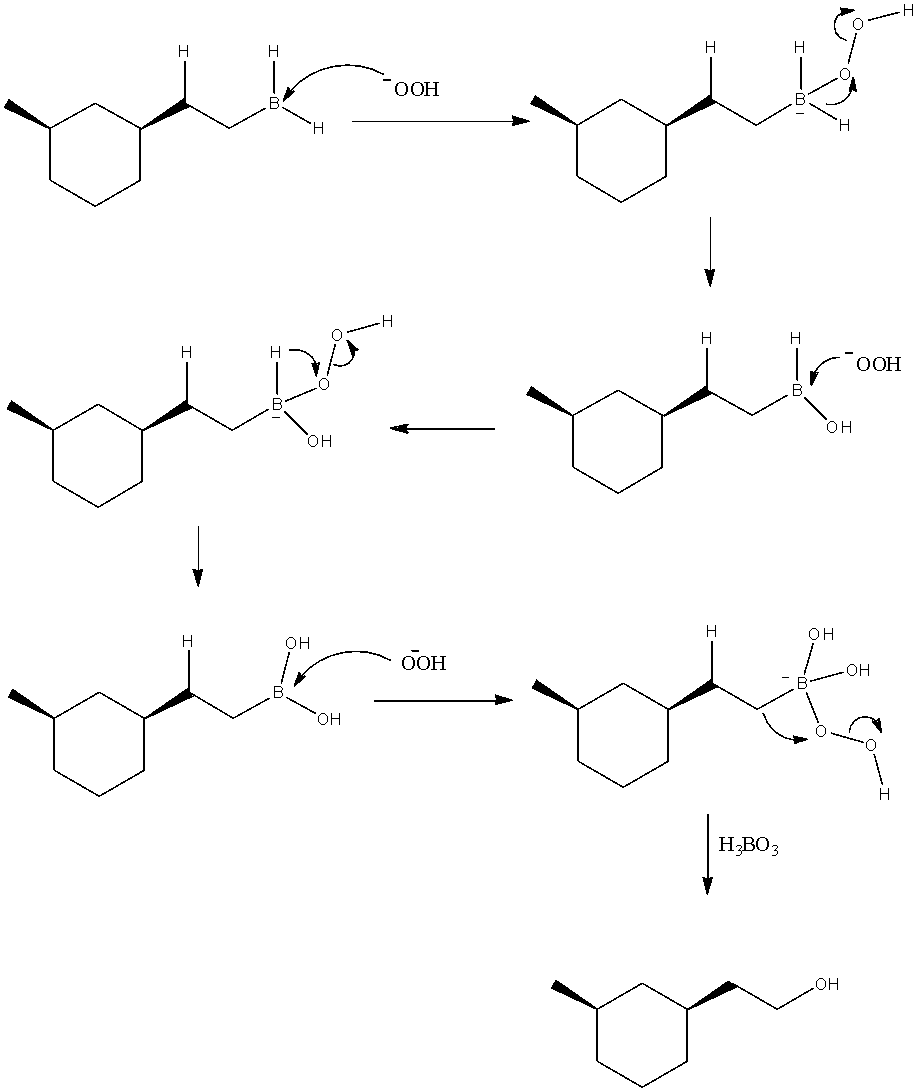
Thus, we can see that upon three cycles of attack of peroxide ions, finally we obtain H3BO3 and the corresponding alcohol of the organic compound.
Thus, we can conclude that the answer of the question is (B).
Note:
Note that in hydroboration reaction, the hydrogen atom and hydroxyl group in the final product is syn to each other. Thus, this is syn-addition reaction. The product is the same as the anti-markovnikov addition product.
