Question
Question: What is the product of the aldol condensation between cyclopentanone and 4-methylbenzaldehyde?...
What is the product of the aldol condensation between cyclopentanone and 4-methylbenzaldehyde?
Solution
We have to remember that the aldol condensation is a natural response where an enolate particle responds with a carbonyl compound to shape a β−hydroxyaldehyde or β−hydroxyketone. Hydroxide capacities as a base and consequently, moves the acidic alpha-hydrogen delivering the responsive enolate particle. This response can be viewed as a corrosive base response. The aldehyde is assaulted at the electrophilic carbonyl carbon by the nucleophilic enolate particle. This assault is a nucleophilic expansion response and gives alkoxide middle. The alkoxide deprotonates water atoms, subsequently creating hydroxide and the β−hydroxy aldehyde.
Complete step by step answer:
We have to know the aldol would then be able to dry out to shape a α,β− unsaturated carbonyl compound. The interaction requires a functioning methylene nearby a carbonyl gathering. On the off chance that there are two dynamic methylene the buildup can happen on each side of the carbonyl gathering.
Now, the product of the aldol condensation between cyclopentanone and 4−methylbenzaldehyde is (2E,5E)−2,5−bis(4−methylbenzylidene)cyclopentanone
The structure of (2E,5E)−2,5−bis(4−methylbenzylidene)cyclopentanone has to be drawn below,
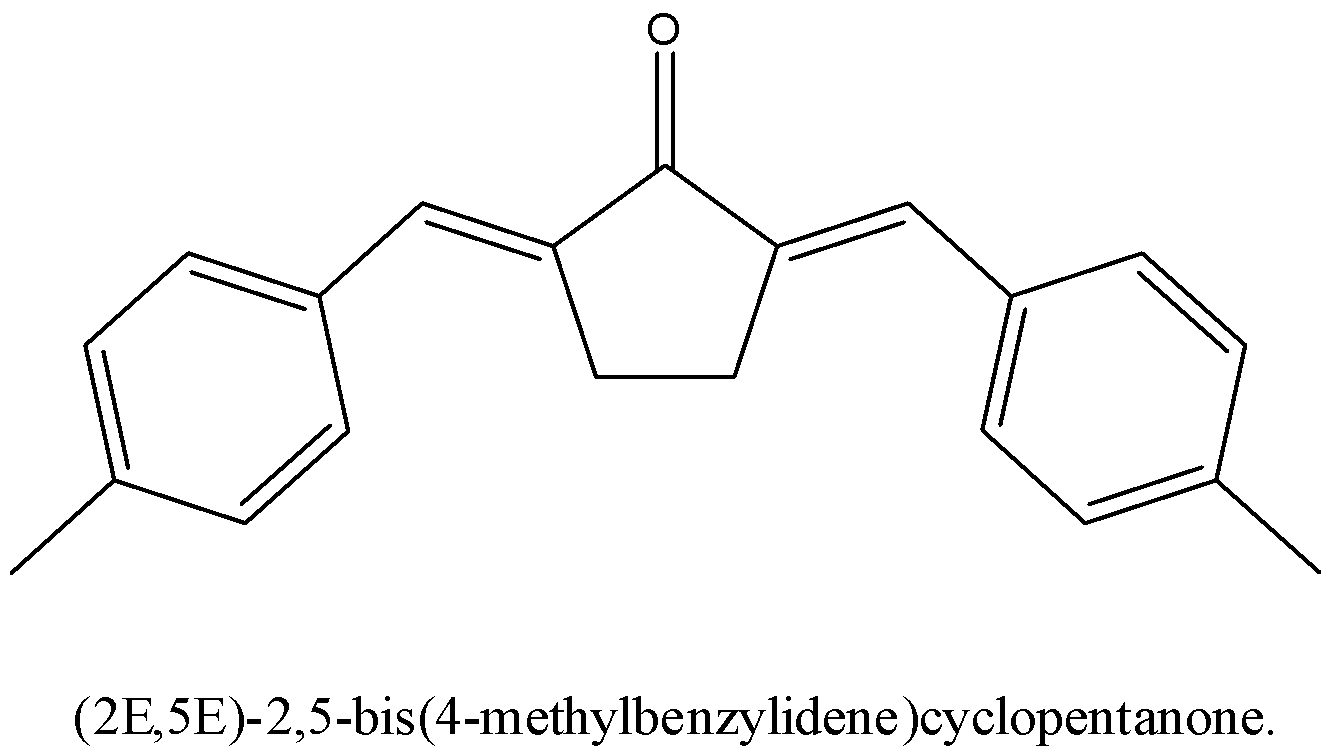
We have to see the E,E isomer ought to be highly steady, in light of the fact that the steric connections of the ortho hydrogens with the hydrogen atom on the cyclopentane ring are more modest than the carbonyl oxygen.
Note: We have to know that within the sight of a suitable base, acetone can go through self-aldol buildup to an alpha-beta unsaturated ketone. The base will respond with acetone to create an enolate, which will to another particle of acetone to achieve the self-aldol buildup.
Formaldehyde does not contain alpha hydrogen particles. Consequently, it does not go through aldol buildup response.
