Question
Question: What is the product obtained in the following reaction? 
A) 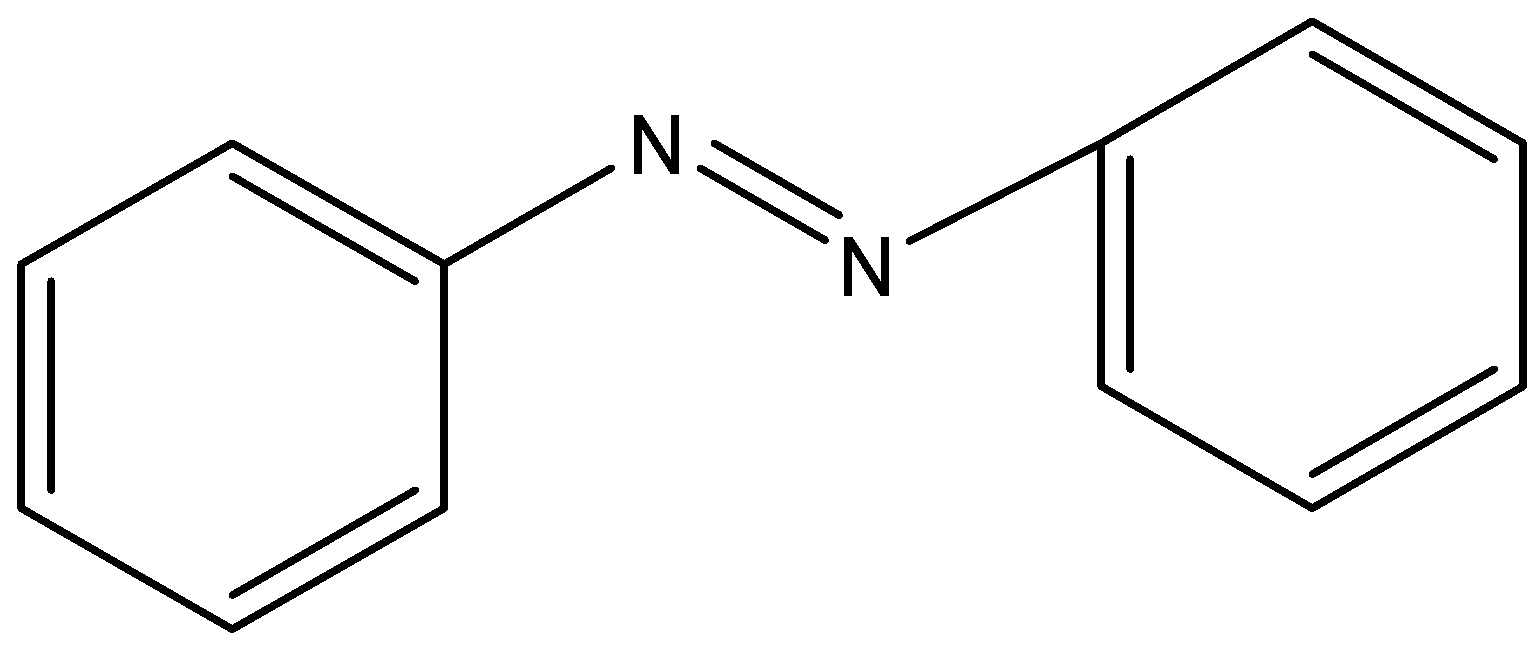
B) 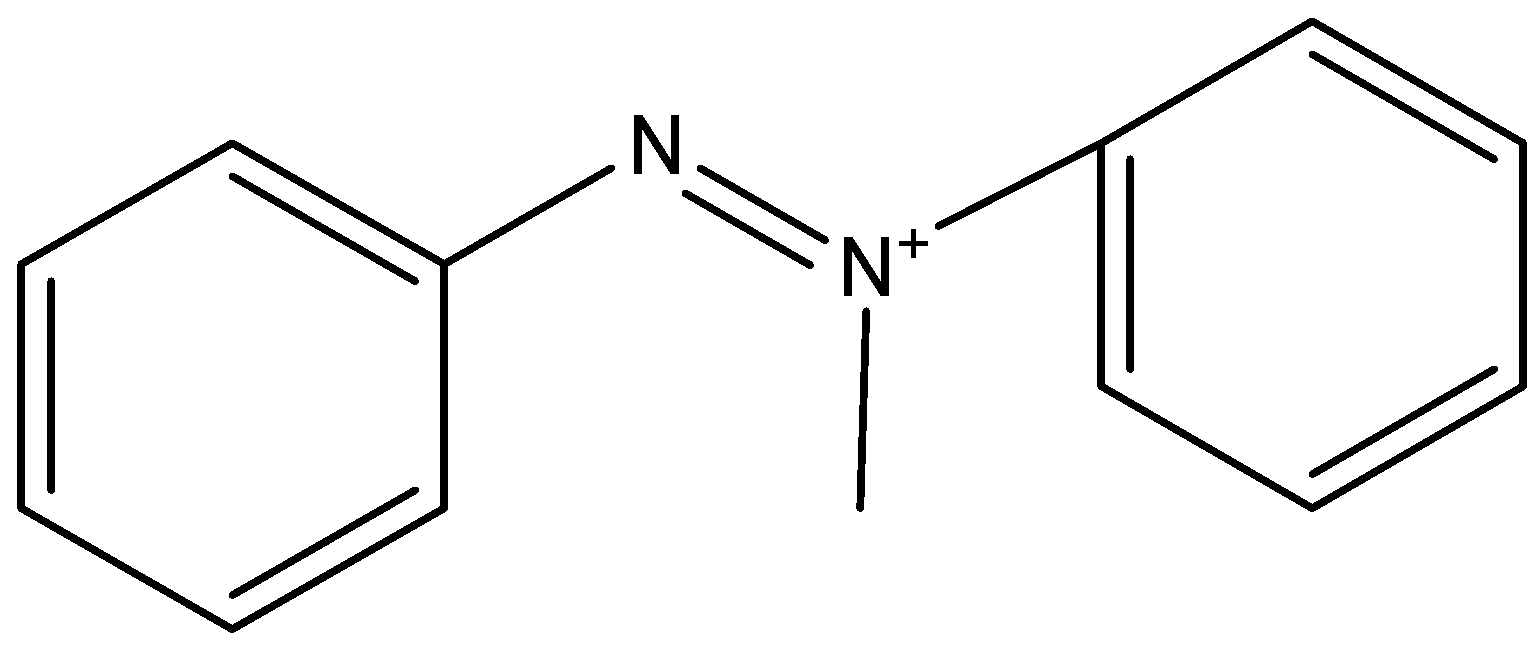
C) 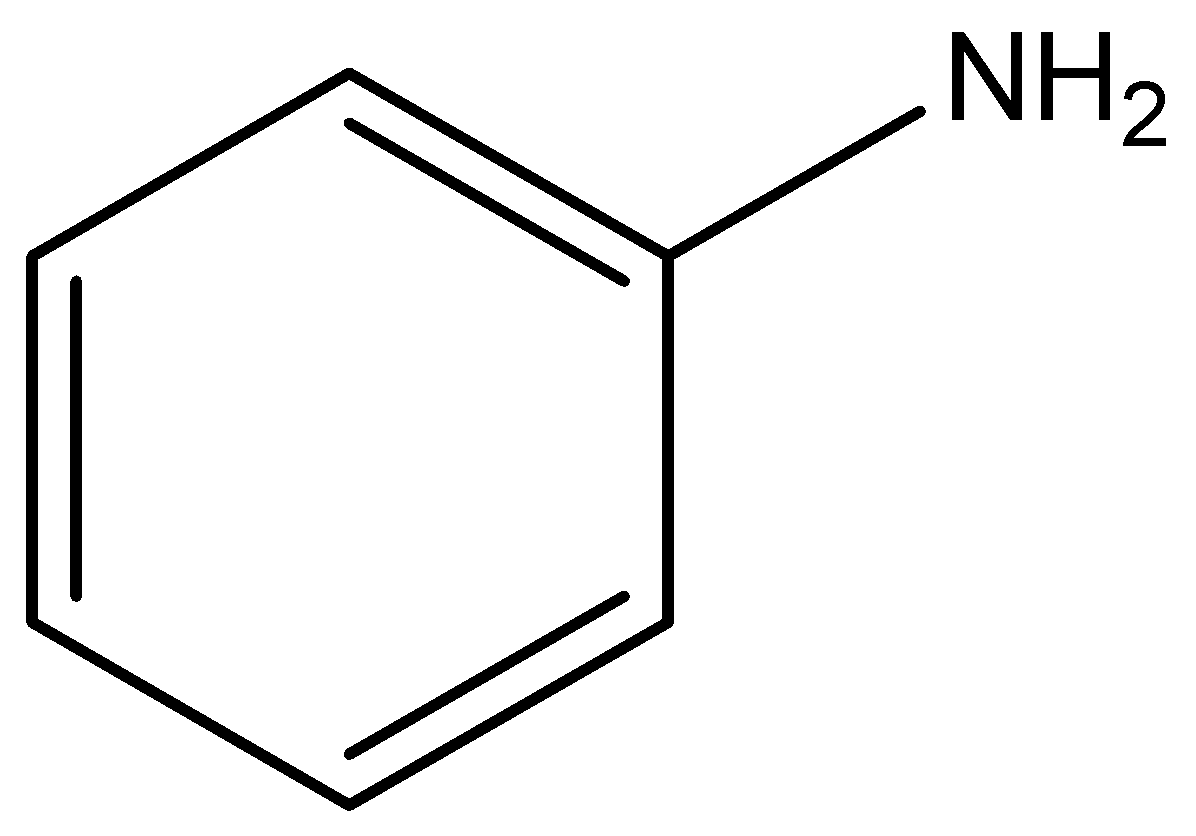
D) 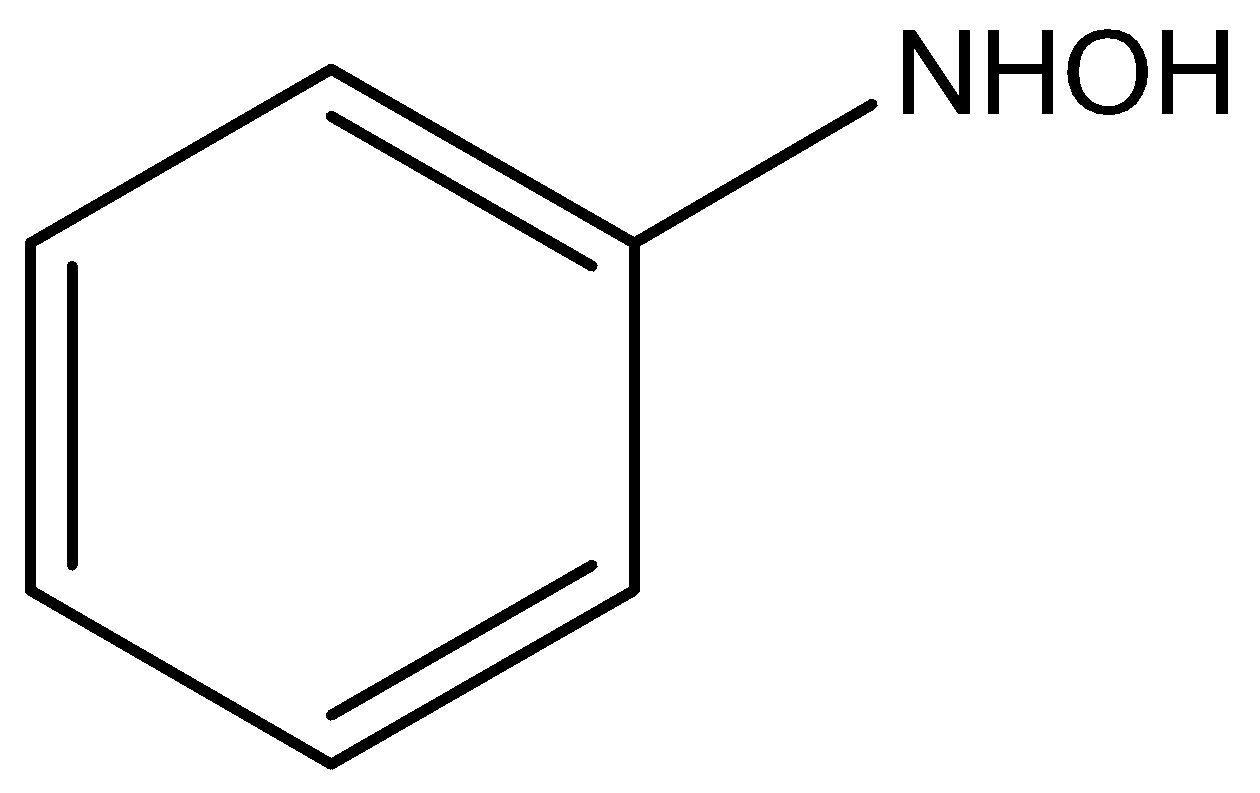
Solution
As we know that in organic chemistry benzene is one of the important compounds. It is one of the aromatic compounds. The molecular formula of benzene is C6H6. Bromobenzene is one of the derivatives of benzene. Nitro benzene is one of the electrophilic substitution compounds in benzene.
Complete answer:
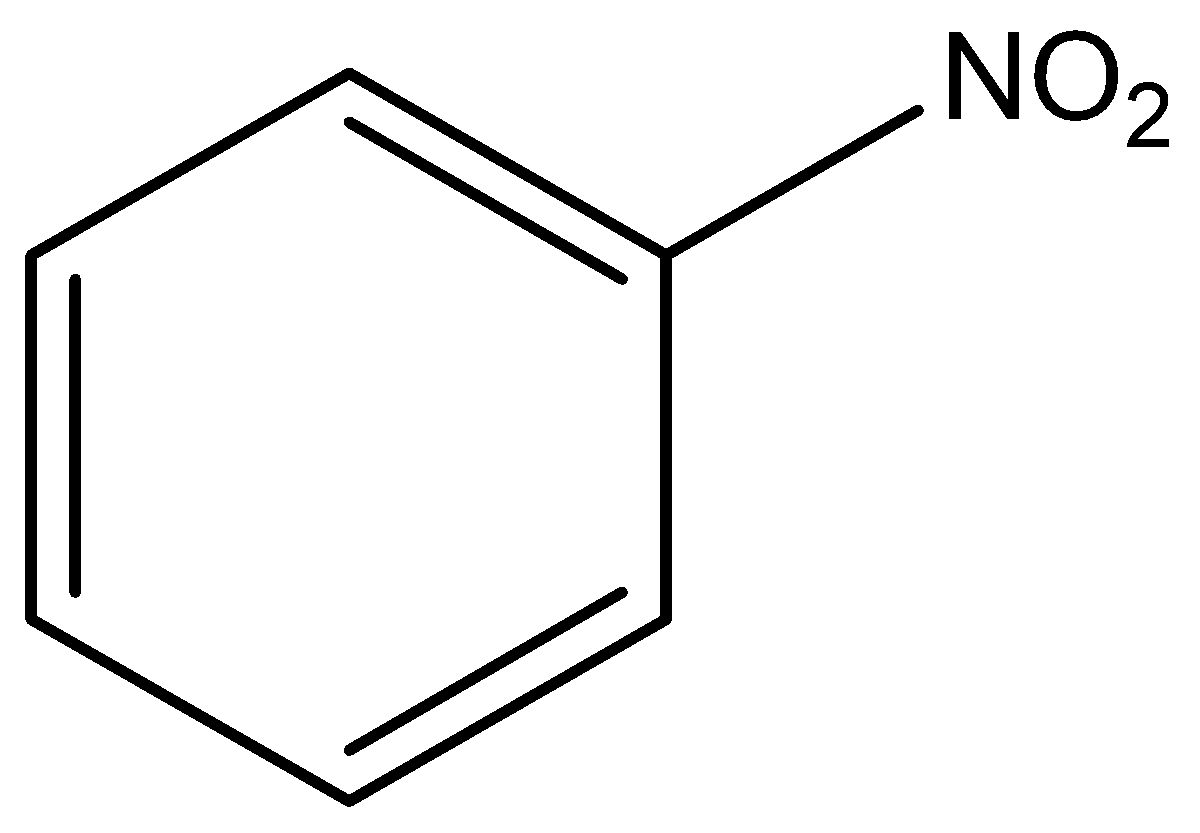
The molecular formula of nitro benzene is C6H5NO2.
The structural formula of nitro benzene is
In this reaction, zinc acts as the metal catalyst. The zinc reaction will move faster than normal.
The molecular formula of ammonium chloride is NH4Cl.
The nitrobenzene reacts with ammonium chloride in the presence of zinc to act as the catalyst to give the product of N-hydroxy benzamide.
The chemical reaction for the above discussion is given below,
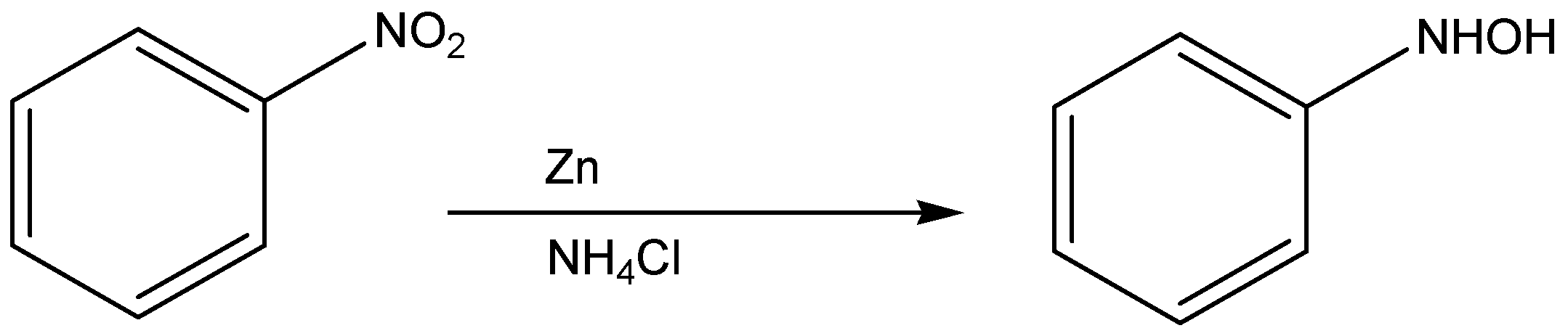
According to the above discussion, we conclude nitro benzene compounds react with ammonium chloride in presence of zinc and act as the catalyst to give the product of N-hydroxy benzamide compound.
Hence, option B is the correct answer.
Note:
We need to remember that nitro benzene is one of the mono substituent benzene compounds. Each mono-substituent moiety has three named positions in the ring. In the ring a nearby mono substituent group is called ortho position, that means two ortho positions are in the ring, because of the left and right side of the substituent group. The alternatively position of the substituent group in the ring is called meta position, here also two meta positions possible in the left and right side of the substituent group. Para position is nothing but directly opposite to the substituent group in the ring. Electrophilic substitutions are attacked in ortho and para position in the ring.
