Question
Question: What is the product E in the following reaction? 
Solution
Here the sequence of reactions takes place. To solve such problems we have to start from the first product and then follow the steps and then predict the final product. There are various reagents in this sequence which behave in different manners. Solve them step by step.
Complete answer:
There is a sequence of reactions that takes place. Each forward reaction is connected to its preceding product. Thus we will solve each product and then reach the final product E.
Formation of A:
Methyl magnesium bromide is made to react with cyclopentanone to give product A. Methyl magnesium bromide is a Grignard reagent. Since cyclopentanone contains carboxyl group, therefore it will be an addition reaction as,
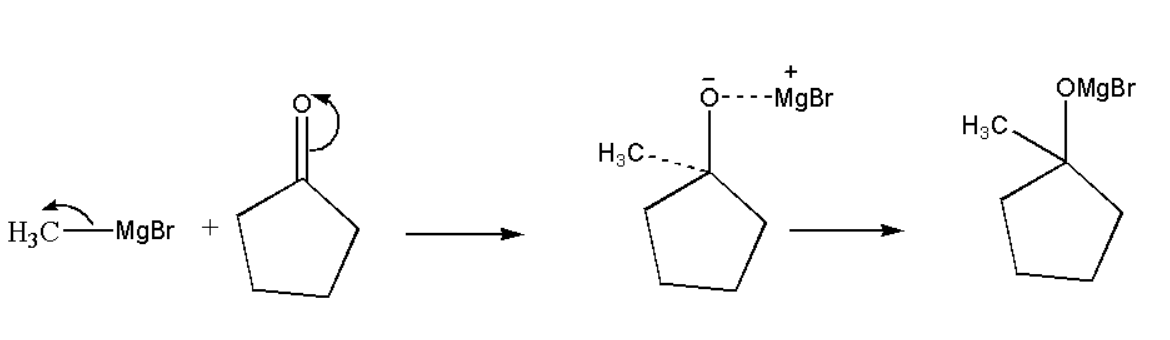
Now the formed product will react with H3O+ to form product A. This reaction is also known as hydrolysis reaction.
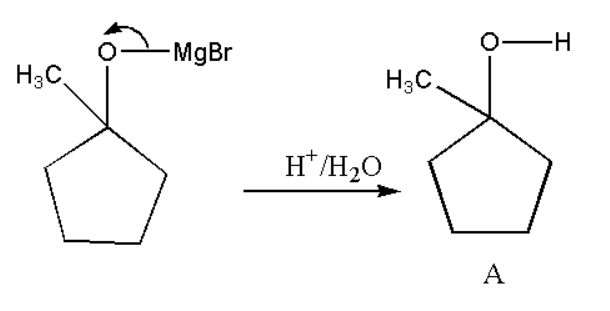
Formation of B:
Now there will be replacement of the hydroxyl group with bromine when HBr is added to A.
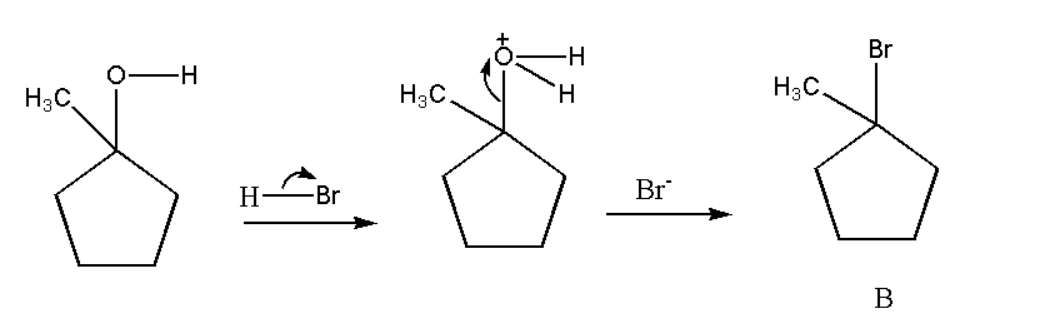
Formation of C:
Since there is presence of bromine atom on the ring and when it react with magnesium in presence of dry ether it will again become a Grignard reagent as,
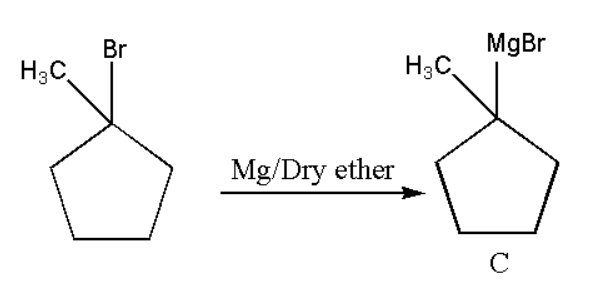
Formation of D:
Now the product C will react with formaldehyde in the presence of H3O+ which give us the following product,
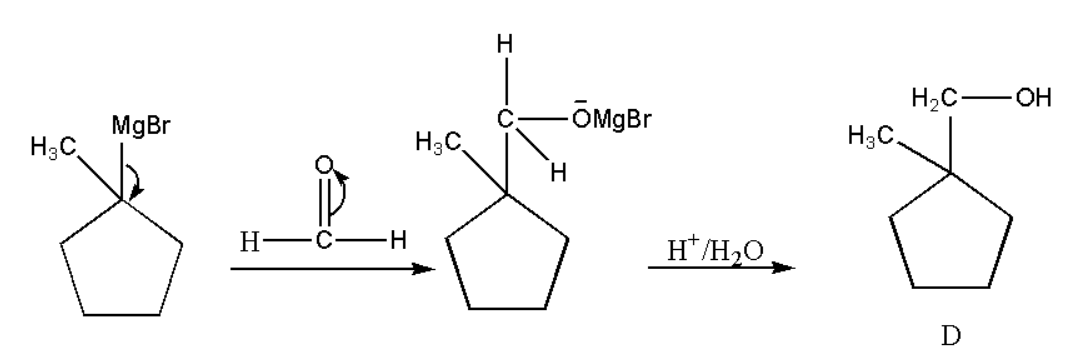
Formation of E:
Now when alcohol react with hydrogen iodide then it forms an alkyl halide which can be shown as,
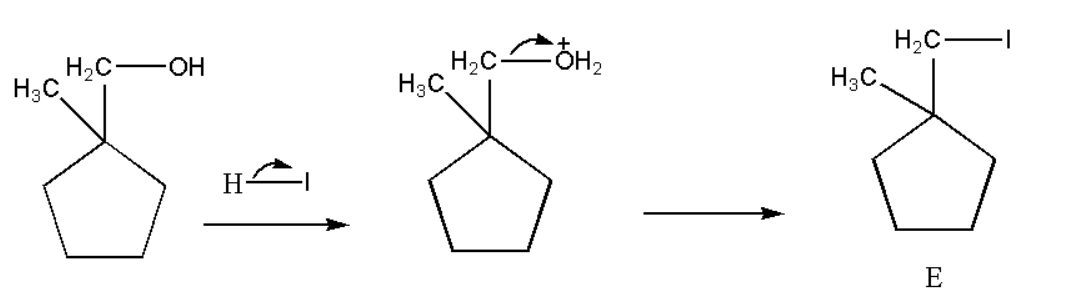
Hence the product formed E is identified.
Note:
We cannot skip any step to find the product E. We cannot directly find the termination step as this would give us the wrong answer. Hydrogen halides when reacted with alcohol give alkyl halides. Here alkyl iodide is formed. By understanding the mechanism of each reaction we can easily understand the product formation of each step.
