Question
Question: What is the product C in the following reaction? 
Solution
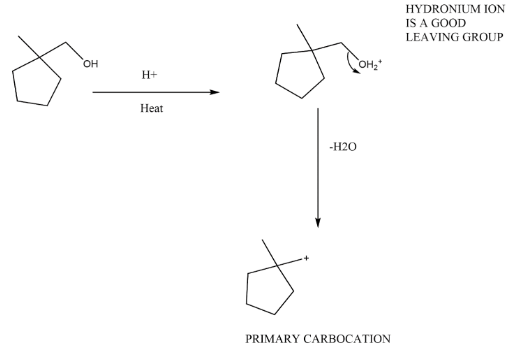
Whenever acidic medium is given with a hydroxyl group, it is added due to make the hydroxyl group as leaving group. In the first step start the reaction with the formation of hydronium ionH2O+ which is very good leaving group. As leaving group leaves it will make a carbocation at its place. Make the rearrangement as possible to make a stable carbocation. Ozone and zinc oxide are given which are for ozonolysis.
Complete step-by-step answer: We have given a five membered ring and an alcohol group such that the group will changes from hydroxide to hydronium ion. This makes the hydroxide a very good leaving group because at its place now H2O+ ion came. Now as 1∘Carbocation forms it will rearrange and changes to 3∘Carbocation and a hydrogen ion further leaves and forms a double bond at its position.
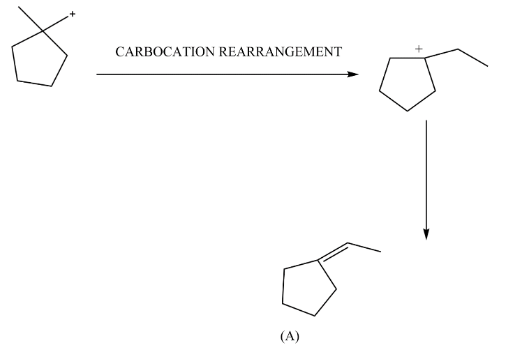
Here, after the formation of (A) there will be ozonolysis by which we get (B).
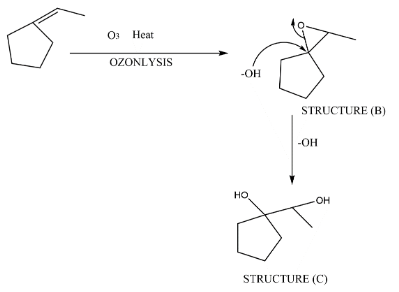
In the process of ozonolysis, there is a formation of an epoxide ring in place of double bond. As you are seeing in the above representation of mechanism, there will be an epoxide ring form. In the next step the hydroxide will attack on the carbon atom by which the epoxide ring will get open and that’s how we get our desired product.
Note: The formation of carbocation is the main step that you want to predict because carbocation is a reaction intermediate and rearrange in such a way that after the reaction a more stable carbocation will form. This is the driving force of some reactions as above the carbocation rearranges from primary to tertiary which is more stable.
