Question
Question: What is the product B in the following reaction: 
A.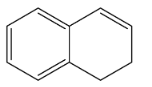
B.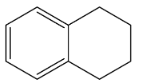
C.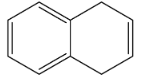
D.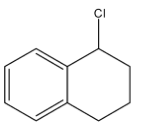
Solution
TsCl is called p-toluenesulfonyl chloride. TSCl reacts with primary or secondary alcohols and forms tosylate. These tosylates are going to act as good leaving groups in the presence of strong reducing agents.
Complete step by step solution:
- In the question it is given to find the product B in the given chemical reaction.
- The given chemical reaction contains two steps.
Step-1:
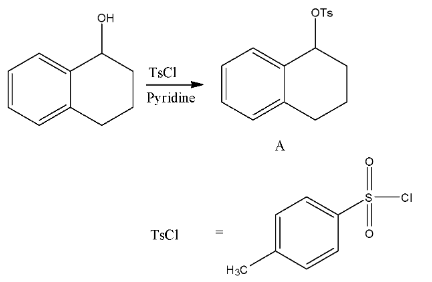
- In the first step TsCl = p-toluenesulfonyl chloride reacts with secondary alcohol in the presence of pyridine as a base and forms tosylate derivative (A).
- TsCl is going to make the alcohol as tosylate.
- We know that tosylate groups are good leaving groups in the presence of strong reducing agents.
Step-2 :
- In the second step the formed tosylate is going to react with lithium aluminum hydride and forms an alkane as a product.
- The chemical reaction of reducing tosylate into alkanes is as follows:
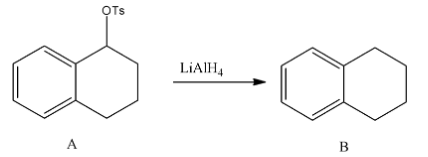
- Means secondary alcohol is going to convert into alkane by a two-step process.
- Therefore the product is alkane.
So, the correct option is B.
Note: In industries this method of preparation of alkanes is most popular because there is no need to use a large number of strong reagents. By using simple reagents like tosyl chloride and lithium aluminum hydride we can prepare alkanes from primary or secondary alcohol. P-toluenesulfonyl chloride is going to prepare from p-toluene sulfonic acid.
