Question
Question: What is the \(pH\) range in which phenolphthalein is colorless? A) \(0 - 8\) B) \(8 - 10\) C) ...
What is the pH range in which phenolphthalein is colorless?
A) 0−8
B) 8−10
C) 10−12
D) 12−14
Solution
We know that Phenolphthalein is an acid-base indicator. An acid-base indicator is also called a pH indicator. Indicators are usually a substance which changes its color with change in pH of the solution.
Complete step by step answer:
We can draw the structure of phenolphthalein as,
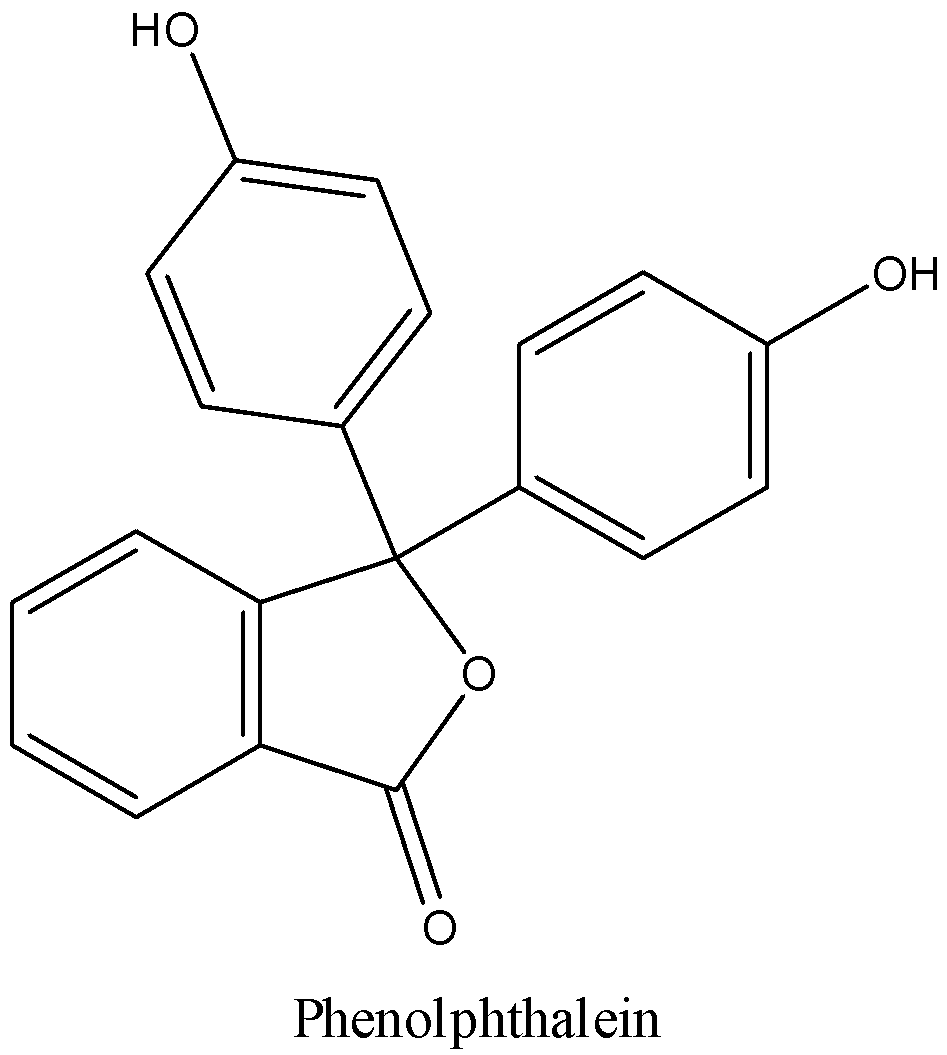
We know that the molecular formula of Phenolphthalein is C20H14O4. It is a weak acid. It is a yellow crystalline solid and easily dissolves in alcohols and slightly soluble in water.
Acid-base indicators are generally weak acids or bases which dissociate when dissolved in water and form ions.
Now, consider a weak acid indicator with the formulaHIn, it dissociates in water as follows.
HIn(aq)+H2O⇌H3O+(aq)+In−(aq)
Acid(ColorA) Conjugatebase(colorB)
The dissociation of Phenolphthalein in water is,
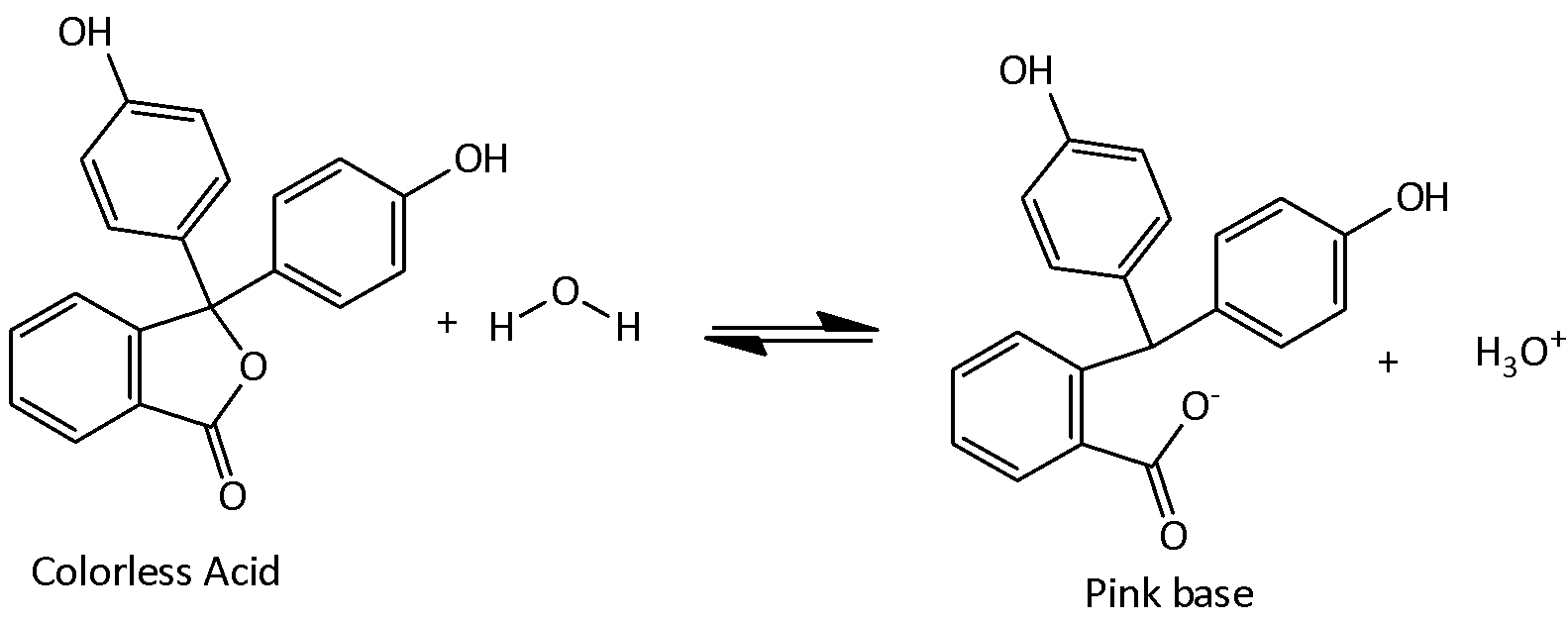
Phenolphthalein indicator used in a solution which changes its color at higher pH. If a weak acid is titrated against a strong base the solution changes its color when the pH solution is greater than 7. Phenolphthalein remains colorless in the acidic pH levels and it turns to pink at pH=8.2 and continues to a bright magenta at pH=10. The color change is due to the ionization of the phenolphthalein which in turn changes the shape of the molecules.
Therefore, the correct option is A. .
Note: We know that pH is the concentration of hydrogen ion in the solution.
The mathematical expression of pH is,
pH=log[H+]
A solution which has pH less than seven then is considered as acidic and a solution which has pH greater than seven then it is considered as basic.
