Question
Question: What is the percentage of carbon, by wt. in vitamin C ? Options- (A) 66.67% (B) 40.9% (C) 20...
What is the percentage of carbon, by wt. in vitamin C ?
Options-
(A) 66.67%
(B) 40.9%
(C) 20%
(D) 60%
Solution
Draw the expanded structure of vitamin C. Calculate the total mass of the compound. Now identify the number of carbon atoms in vitamin C. Calculate their total mass. Now take the ratio of mass of carbon atoms and total mass of compound. Now multiply this with 100 to obtain the correct answer.
Complete answer:
Vitamin C also called as ascorbic acid has the following structure.
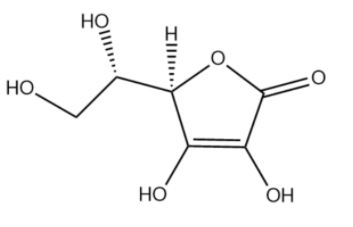
We will now determine the total mass of the above compound.
Total number of carbon atoms = 6
Total number of oxygen atoms = 6
Total number of hydrogen atoms = 8
Mass of 1 carbon atom = 12 g
Mass of 1 oxygen atom = 16 g
Mass of 1 hydrogen atom = 1 g
Total mass of the compound = (6 x 12) + ( 6 x 16) + ( 8 x 1) = 176 g
Mass of carbon atoms = 72 g
The ratio of mass of carbon atoms and total mass of compound gives the value, 0.4099
Hence the percentage of carbon, by wt. in vitamin C is 0.4099 x 100 = 40.9%
Therefore, the correct answer is option (B).
Note:
It is important to know that the structure of ascorbic acid does not have any carboxylic acid group. It is called an acid due to its pH value mainly. One more organic compound like this is picric acid which too is called an acid due to its pH value.
