Question
Question: What is the packing efficiency for an end centered unit cell?...
What is the packing efficiency for an end centered unit cell?
Solution
Hint- The packing efficiency can be calculated by the percent of space occupied by spheres present in a unit cell. Here we will proceed further by evaluating the volume of spheres in the unit cell and total volume of the unit cell.
Complete answer:
We will use the following figure of the end centered unit cell to solve the problem.
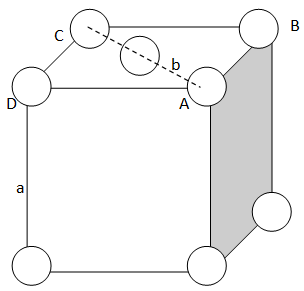
Let the side of an unit cell = a
And diagonal AC = b
Now, in right triangle ABC,
Let us use the Pythagoras theorem.
AD is perpendicular, DC is base and AC is diagonal
∵AC2=AD2+DC2 ⇒b2=a2+a2 ⇒b2=2a2 ⇒b=2a
Let r is the radius of sphere, so b = 4r,
Thus,
Now, volume of cube =(side)3=a3
Substituting the value of a from equation (i) we get,
Volume of cube
=a3=(22r)3 =8×22×r3 =162r3
Volume of cube =162r3 ---- (2)
Now, volume of sphere
=34πr3..........(3)
Since one unit cell of end entered cell has 2 spheres
=81×8+21×2=1+1=2
As the contribution of the corner sphere in a cell is one eighth and that of the face sphere is half.
Therefore, volume of 2 atoms, i.e. 2 spheres:
=2×34πr3 =38πr3
We know that
Packing efficiency = (Volume of spheres in unit cell/ total volume of unit cell) × 100%
Since there are 2 atoms in the unit cell of end centered cell
Therefore, packing efficiency of end centered cell
Packing efficiency = (Volume of 2 spheres in unit cell/ total volume of unit cell) × 100%
Now, packing efficiency (in %)
=total volume of unit cellvolume of 2 spheres in unit cell×100 =162r338πr3×100
Let us solve the equation by cancelling the common term to find the percentage.
=3×1628π×100 =3×22π×100 =3×1.4143.14×50 =37.02%
Hence, packing efficiency for end centered unit cell is 37.02%
Note- Packing efficiency is defined as the percentage of space occupied by constituent particles packed inside the lattice. It can be calculated with the help of geometry in three structures namely: HCP and CCP structures. The packing efficiency of simple cubic lattice is 52.4%. And the packing efficiency of body centered cubic lattice (bcc) is 68%.
