Question
Question: What is the oxidation state of Sn in $SnSO_4$?...
What is the oxidation state of Sn in SnSO4?
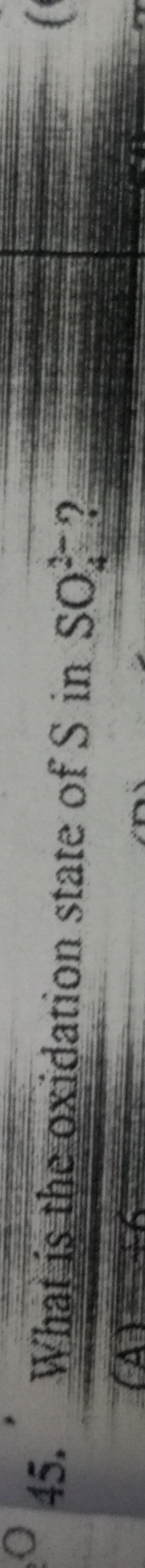
Answer
+2
Explanation
Solution
Let the oxidation state of Sn be x.
For the sulfate ion, SO42−:
- Oxidation state of S = +6
- Each O = −2 and there are 4 O atoms: 4(−2)=−8
- Sum for sulfate: +6−8=−2
Since the compound is neutral:
x+(−2)=0⟹x=+2