Question
Question: What is the oxidation state of Chromium (Cr) in \({\rm{Cr}}{{\rm{O}}_{\rm{5}}}\)....
What is the oxidation state of Chromium (Cr) in CrO5.
Solution
Oxidation state is the number of electrons that an atom needs to lose or gain to result in a chemical bond. To know the oxidation state of CrO5, first we have to draw its structure so that we identify the type of oxygen atom.
Complete step-by-step answer:
The structure of CrO5is,
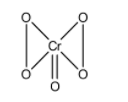
In the compounds, four oxygen atoms are part of peroxide linkage and we know that in peroxides the oxidation state of oxygen is -1. And in all other compounds, the oxidation state of oxygen atom is -2.
If we know the type of compound, we will easily know the oxidation number of compounds. For neutral compounds, such as H2O, CrO5, etc. the addition of oxidation state of all atoms of the compound is equal to zero.
So, in the compound, CrO5, four peroxide oxygen atoms has oxidation state of -1 each and the remaining oxygen has oxidation state of -2. Using these values, now we calculate the oxidation state of chromium.
We take x as the oxidation state of chromium in CrO5.
x+(−1×4)+(−2)=0\x−4−2=0\x=6
Hence, the oxidation state of chromium in CrO5 is 6.
Additional Information:
- If a compound exists in elemental form (only one type of atoms present), the oxidation number of the element is always zero.
- For ions, the charge indicates the oxidation number. For example, the oxidation number of chloride ions is -1.
Note: Students have to take care of taking the oxidation number of oxygen in the calculation of an oxidation number of an element in a compound. In peroxides, oxygen possesses oxidation state of -1. In CrO5 four oxygen atoms has -1 oxidation state and the remaining one oxygen atom possesses oxidation state of -2.
