Question
Question: What is the oxidation number of 'S' bond with oxygen in $S_4O_6^{2-}$ ion? $4x - 2 \times 6 = -2$...
What is the oxidation number of 'S' bond with oxygen in S4O62− ion? 4x−2×6=−2
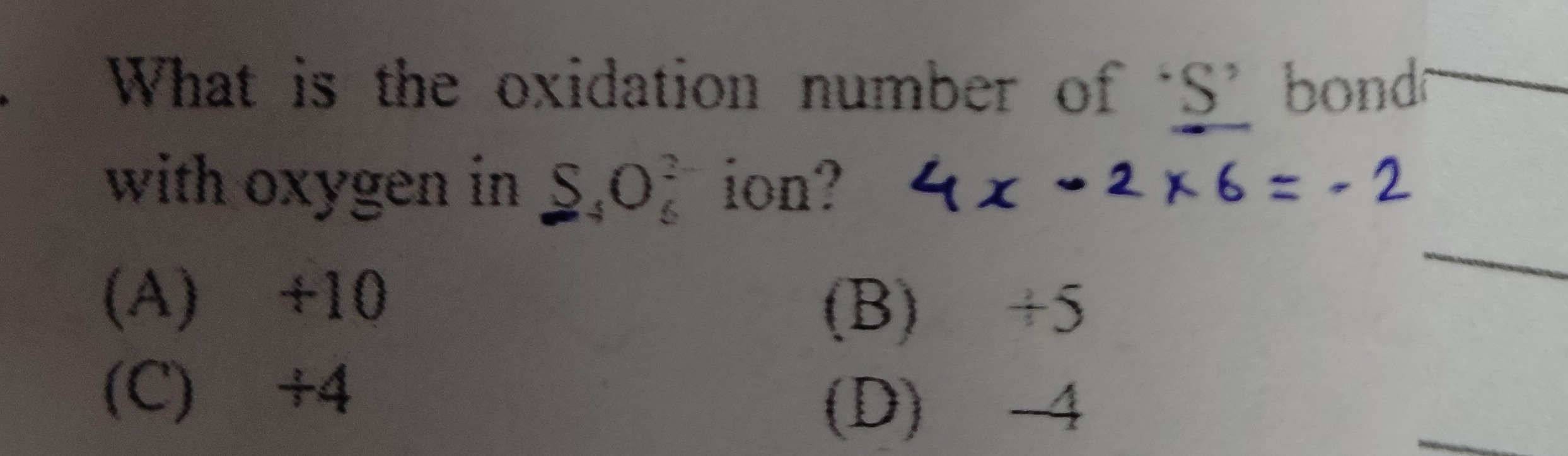
A
+10
B
+5
C
+4
D
-4
Answer
+5
Explanation
Solution
In the tetrathionate ion, S4O62−, the overall oxidation numbers sum to −2. If we assume each sulfur had the same oxidation state x, then:
4x+6(−2)=−2⟹4x−12=−2⟹4x=10⟹x=+2.5This is the average oxidation number of sulfur.
However, the tetrathionate ion actually contains two different types of sulfur atoms:
- Terminal S atoms (bonded to oxygen): These are part of sulfonate (−SO3) groups and hence have oxidation states similar to those in sulfate, i.e., +5.
- Central (bridging) S atoms: Their oxidation number adjusts so that the overall average becomes +2.5. (Here, each central S comes out as 0.)
Thus, the sulfur atoms that are bonded to oxygen (the terminal ones) have an oxidation number of +5.
