Question
Question: What is the oxidation number of \(Cr\) in \[{(N{H_3})_3}Cr{O_4}\]or \[{(N{H_3})_3}Cr{({O_2})_2}\]?...
What is the oxidation number of Cr in (NH3)3CrO4or (NH3)3Cr(O2)2?
Solution
Oxidation Number of an element or compound will depend on the number of electrons that are gained or lost by an atom that is present in a compound. Oxidation number is also known as the Oxidation state of a compound. We can determine the Oxidation number of the element by following certain rules.
Complete step by step solution:
Let us consider of the compound (NH3)3CrO4 or (NH3)3Cr(O2)2
The structure of (NH3)3CrO4 or (NH3)3Cr(O2)2 is a pentagonal bipyramidal. The structure is given below.
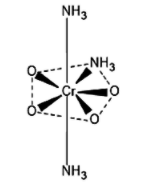
Let us now calculate the oxidation number of Cr in (NH3)3CrO4 or (NH3)3Cr(O2)2.
Consider the oxidation number of Chromium as x
The total oxidation number of the compound is 0.
The oxidation number of the NH3 group is 0.
Therefore, 3×0=0
The oxidation number of Oxygen is -1. Because the oxygen present in (NH3)3CrO4 is in the peroxide group.
Therefore,
3×0+x+4×−1=0
x−4=0
x=4
Therefore, the oxidation number of Cr in (NH3)3CrO4 or (NH3)3Cr(O2)2 is +4.
Additional information:
We have to follow certain rules to assign the oxidation number to certain elements.
- Atoms that are in the free elemental state i.e. (H2,O2) will be 0.
- Monatomic ions Na+=1
- Halogen like Cl−=−1
- Oxygen =-2
- Hydrogen =1
- Metal hydrides -1.
Note: We must always remember that the oxidation state of the oxygen atom in peroxide will always be -1, as the oxygen atom is linked to only the chromium atom through a single bond. The Oxygen which does not belong to the peroxide molecule will be having the oxidation number of -2.
