Question
Question: What is the other name of the process in which alkaline hydrolysis of ester occurs? A. dehydrogena...
What is the other name of the process in which alkaline hydrolysis of ester occurs?
A. dehydrogenation
B. dehydration
C. esterification
D. saponification
Solution
We know that an ester is a chemical compound formed when alcohol reacts with a carboxylic acid.
We also know that an alkaline solution is the solution of a base and water. During the alkaline hydrolysis, the nucleophilic substitution of OH− occurs at −C=O. This reaction produces carboxylic salt and water.
Complete answer:
We know that an ester is a chemical compound formed when alcohol reacts with a carboxylic acid. This process is called esterification. In this water molecule gets eliminated, therefore the type of reaction is a condensation reaction. It is a reversible process.
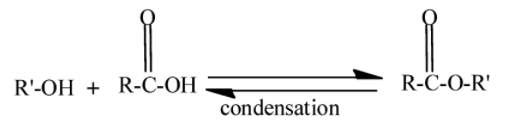
Here −R′− and −R− are alkyl groups. Hydrolysis is the process in which the water molecules are used to break the chemical compound. Alkaline solutions are the solutions of water and bases. Bases like NaOH,KOH etc. Bases are the compounds which give OH− in water. So when ester undergoes hydrolysis in an alkaline solution it gives carboxylic salt and water.
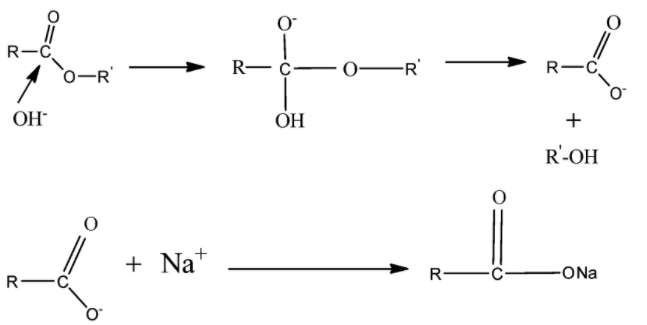
Here NaOH breaks into Na+ and OH− ions. OH− acts as a nucleophile that is an electron-donating species and causes substitution reaction at −C=O . Alkaline hydrolysis of ester is also known as Saponification.
**Therefore, the correct option is D. saponification
Note:**
It is important to note that the hydrolysis of an ester is of two types. One is hydrolysis in an acidic medium and the other is hydrolysis in an alkaline medium. Acidic medium is the solution of an acid and water. The products in both mediums are different. In acidic hydrolysis, we get carboxylic acid and alcohol as products.
