Question
Question: What is the order of stability for I) I-butene II) cis-2-butene III) trans-2-butene A) \(...
What is the order of stability for
I) I-butene
II) cis-2-butene
III) trans-2-butene
A) I < II < III
B) I > II > III
C) III > I > II
D) I > II > III
Solution
Hyperconjugation is one of the phenomena which stabilizes the molecule.
The cis and trans butane are the geometrical isomers of butane.
Complete step by step answer:
Here in the question, the stability order of the given compounds should be compared for getting the answer.
For that we should know a phenomenon called hyperconjugation.
Hyperconjugation is the no bond resonance phenomenon in which the compound stabilizes itself by attracting the electrons from the alpha hydrogen to the nearest carbon.
Alpha hydrogen is the hydrogen of the nearest C with respect to which we speak about the phenomenon.
As the number of alpha hydrogen in the structure is more, more is the hyperconjugation structure and more stable the structure is.
- Now let’s consider the options and comment on its stability.
The options given here is - I-butene, cis-2-butene and trans-2-butene
Cis and trans butane are the isomers of butene.
Consider the structures of the three compounds.
First let’s see the structure of 1-butene,
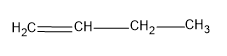
It has only two alpha hydrogens, so only two hyperconjugations are possible for this structure. Hence the stability of this structure will be low.
Now let’s consider the structure of cis-2-butene.
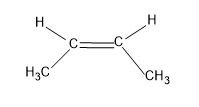
Here there are six alpha hydrogens which are attached to the carbons but the methyl group is present in the same side which causes steric hindrance.And steric hindrance decreases the stability of the compound.
The last option is trans-2-butene
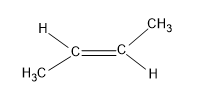
Here also six alpha hydrogens are present but the methyl group is present on the opposite side of the carbon, hence it stabilizes the structure.
So we can conclude that, as in 1-butene there is only two alpha hydrogen present, it will be the least stable structure. And comparing cis and trans isomers, cis will have the least stability since there is steric hindrance in the structure and trans-2-butene will be the most stable structure among the given options,
Hence the correct option is option (A). I < II < III
Note: The more substituted alkene will be the most stable one than the least substituted alkenes. And the least substituted alkenes are more stable than the unsubstituted or normal alkene.
Here the order of stability is, Alkene < substituted alkenes.
Steric effect is another one factor that should be given consideration, while comparing the stabilities of the structure.
